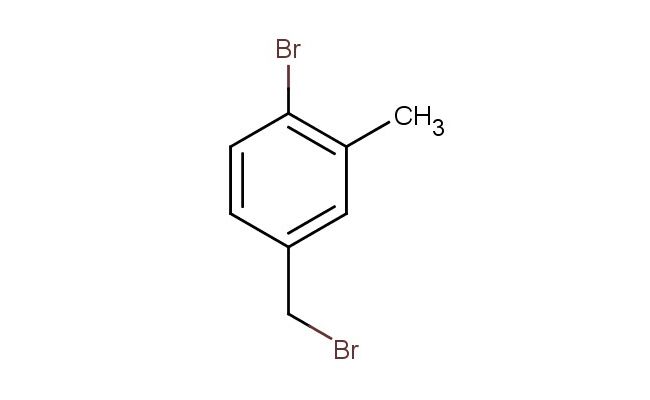
4-bromo-3-methylbenzylbromide
$250.00
CAS No.: 27561-51-9
Catalog No.: 194048
Purity: 95%
MF: C8H8Br2
MW: 263.96
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C=C(CBr)C=C1)C
Catalog No.: 194048
Purity: 95%
MF: C8H8Br2
MW: 263.96
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C=C(CBr)C=C1)C
4-bromo-3-methylbenzylbromide; CAS No.: 27561-51-9; 4-bromo-3-methylbenzylbromide. PROPERTIES: 4-bromo-3-methylbenzylbromide is a colorless to pale yellow liquid with a molecular weight of 257.0 g/mol. It has a boiling point around 185-187 C at 760 mmHg and exhibits low water solubility while being miscible with common organic solvents such as dichloromethane, ethyl acetate, and tetrahydrofuran. The compound contains reactive functional groups including a benzyl bromide group, bromine substituent, and methyl group, which contribute to its chemical versatility and high reactivity. Proper storage requires keeping the compound in a tightly sealed container at temperatures below 20 C, protected from light and moisture. Safety precautions are crucial due to the presence of the benzyl bromide group, which can be highly reactive and corrosive. Protective gloves, eye protection, and adequate ventilation are essential during handling. The compound may cause serious eye damage, skin burns, and respiratory irritation, and appropriate first aid measures should be followed in case of exposure. APPLICATIONS: 4-bromo-3-methylbenzylbromide serves as a versatile alkylating agent in organic synthesis, particularly in the preparation of various pharmaceuticals and specialty chemicals. Its unique substitution pattern makes it suitable for the synthesis of biologically active molecules, including certain antiviral agents and antibiotics, as documented in medicinal chemistry literature. In materials science, the compound functions as a building block for certain types of liquid crystalline materials and conductive polymers. The benzyl bromide group allows for nucleophilic substitution reactions, enabling the introduction of various substituents. Additionally, it is utilized in the preparation of specialty dyes and pigments, where its reactivity facilitates the formation of desired structures. The strategic positioning of the bromine and methyl substituents allows for selective derivatization, expanding its utility in organic synthesis. The compound's high reactivity makes it particularly useful in reactions requiring efficient alkyl group transfer.
Reviews
Write Your Own Review