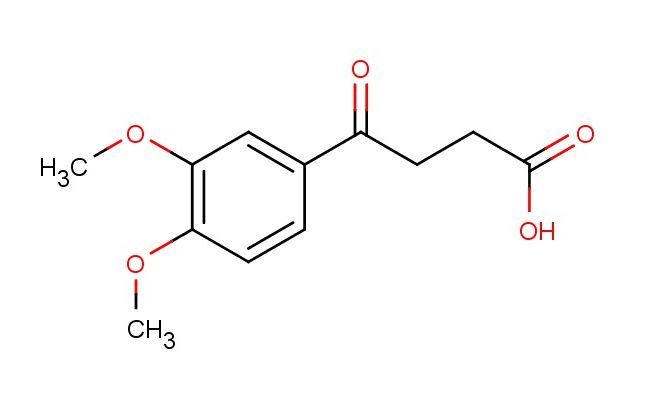
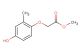
4-(3,4-dimethoxyphenyl)-4-oxobutanoic acid
$200.00
CAS No.: 5333-34-6
Catalog No.: WLZ1189
Purity: 95%
MF: C12H14O5
MW: 238.239
Storage: 2-8 degree Celsius
SMILES: COC=1C=C(C=CC1OC)C(CCC(=O)O)=O
Catalog No.: WLZ1189
Purity: 95%
MF: C12H14O5
MW: 238.239
Storage: 2-8 degree Celsius
SMILES: COC=1C=C(C=CC1OC)C(CCC(=O)O)=O
For R&D use only. Not for human or animal use.
CAS NO.: 5333-34-6; 4-(3,4-dimethoxyphenyl)-4-oxobutanoic acid. PROPERTIES: This aromatic carboxylic acid features a methoxylated phenyl ring connected via a ketone group to a carboxylic acid functionality, creating a molecule with potential antioxidant and anti-inflammatory properties. The 4-(3,4-dimethoxyphenyl)-4-oxobutanoic acid typically appears as a white crystalline powder with moderate aqueous solubility that increases with pH adjustment. Its molecular structure includes a conjugated system between the phenyl ring and ketone, which contributes to its characteristic absorption spectrum. For optimal stability, this compound should be stored at 2-8 degree Celsius in a tightly sealed container away from moisture and direct light. When handling, chemists should wear appropriate personal protective equipment including nitrile gloves and safety goggles. This compound is hygroscopic and may form salts upon exposure to atmospheric moisture. In case of accidental ingestion, rinse mouth thoroughly and seek medical attention. APPLICATIONS: The 4-(3,4-dimethoxyphenyl)-4-oxobutanoic acid serves as a valuable intermediate in the synthesis of bioactive molecules, particularly in the development of anti-inflammatory agents and antioxidants. Its phenolic character makes it suitable for incorporation into compounds targeting oxidative stress-related conditions. In medicinal chemistry, this acid functions as a building block for creating prodrugs designed to improve pharmacokinetic profiles of therapeutic agents. Additionally, the compound finds utility in cosmetic formulations where its antioxidant properties can help stabilize sensitive ingredients and protect against environmental damage. Researchers utilizing this compound benefit from its versatile reactivity, enabling the creation of diverse molecular architectures for drug discovery and development.
Reviews
Write Your Own Review