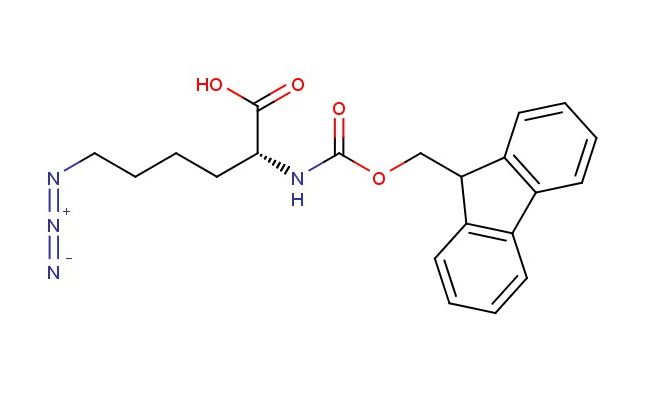
N2-(((9H-fluoren-9-yl)methoxy)carbonyl)-N6-diazo-D-lysine
$300.00
CAS No.: 1198791-53-5
Catalog No.: WLZ1115
Purity: 95%
MF: C21H22N4O4
MW: 394.431
Storage: 2-8 degree Celsius
SMILES: O=C(O)[C@@H](CCCCN=[N+]=[N-])NC(OCC1C2=C(C3=C1C=CC=C3)C=CC=C2)=O
Catalog No.: WLZ1115
Purity: 95%
MF: C21H22N4O4
MW: 394.431
Storage: 2-8 degree Celsius
SMILES: O=C(O)[C@@H](CCCCN=[N+]=[N-])NC(OCC1C2=C(C3=C1C=CC=C3)C=CC=C2)=O
For R&D use only. Not for human or animal use.
CAS NO.: 1198791-53-5; N2-(((9H-fluoren-9-yl)methoxy)carbonyl)-N6-diazo-D-lysine. PROPERTIES: This photoactivatable amino acid derivative combines a fluorenylmethoxycarbonyl (Fmoc) protecting group on the -amino group with a diazo functionality at the -position, creating a powerful tool for light-controlled chemical reactions. The N2-(((9H-fluoren-9-yl)methoxy)carbonyl)-N6-diazo-D-lysine generally appears as a pale yellow crystalline solid with limited aqueous solubility but good solubility in organic solvents like dimethyl sulfoxide and tetrahydrofuran. Its molecular architecture includes a diazo group that can undergo photolytic cleavage upon UV irradiation, releasing the Fmoc-protected lysine residue. For maximum stability and to prevent unintended photoactivation, this compound must be stored at 2-8 degree Celsius in an amber vial wrapped with aluminum foil. When handling, chemists should employ standard laboratory safety practices including nitrile gloves, safety goggles, and a face shield. All manipulations should be performed under subdued lighting conditions to prevent premature activation. In case of skin exposure, wash immediately with soap and water; if eye contact occurs, rinse thoroughly and consult an ophthalmologist. This compound is photosensitive and hygroscopic, requiring careful management of environmental conditions during storage and use. APPLICATIONS: The N2-(((9H-fluoren-9-yl)methoxy)carbonyl)-N6-diazo-D-lysine is extensively used in photopharmacology research where spatial and temporal control over bioactive molecules is essential. Its photoactivatable nature enables the creation of caged compounds that release bioactive species upon targeted light irradiation, facilitating studies on receptor-ligand interactions and signal transduction pathways. In chemical biology, this compound serves as a building block for synthesizing photoresponsive peptides and proteins, allowing researchers to investigate dynamic cellular processes with optical control. The diazo group provides a handle for light-induced uncaging, making this compound valuable for developing photoswitchable biomaterials and smart drug delivery systems. Additionally, the Fmoc protection strategy allows for compatibility with standard peptide synthesis protocols, enabling the incorporation of photoactivatable functionalities into complex peptide architectures. Researchers utilizing this compound can leverage its unique photochemical properties to advance investigations into light-controlled biological systems and develop novel therapeutic approaches based on optical regulation of biomolecular function.
Reviews
Write Your Own Review