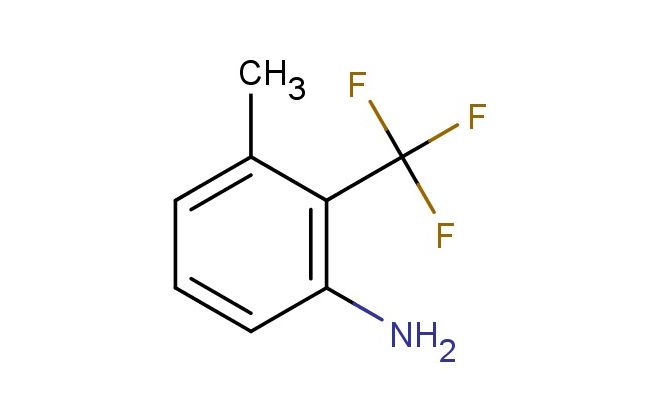
3-methyl-2-(trifluoromethyl)aniline
$300.00
CAS No.: 106877-30-9
Catalog No.: 194021
Purity: 95%
MF: C8H8F3N
MW: 175.153
Storage: 2-8 degree Celsius
SMILES: CC=1C(=C(N)C=CC1)C(F)(F)F
Catalog No.: 194021
Purity: 95%
MF: C8H8F3N
MW: 175.153
Storage: 2-8 degree Celsius
SMILES: CC=1C(=C(N)C=CC1)C(F)(F)F
3-methyl-2-(trifluoromethyl)aniline; CAS No.: 106877-30-9; 3-methyl-2-(trifluoromethyl)aniline. PROPERTIES: 3-methyl-2-(trifluoromethyl)aniline is a halogenated aromatic amine with a molecular weight of approximately 175.2 g/mol. It typically appears as a colorless to pale yellow liquid with a characteristic amine odor. The substance has a boiling point in the range of 120-125 C and a density of approximately 1.15 g/cm?. It exhibits moderate solubility in organic solvents such as diethyl ether, chloroform, and THF, but is sparingly soluble in water. Proper storage requires a cool, dry location in tightly sealed containers, preferably under nitrogen to prevent oxidation. Safety precautions include classification as harmful if swallowed, causes skin irritation, and serious eye damage. It may also cause respiratory tract irritation. Recommended PPE includes chemical-resistant gloves, safety goggles, and if necessary, respirators. Exposure limits typically follow ACGIH TLV guidelines for aromatic amines. APPLICATIONS: 3-methyl-2-(trifluoromethyl)aniline serves as a valuable intermediate in the synthesis of serotonin-norepinephrine reuptake inhibitors (SNRIs), where the amine group participates in forming crucial amine linkages. The trifluoromethyl substituent provides metabolic stability against oxidative degradation. In materials science, the compound is utilized in the preparation of conductive polymers, with the aromatic amine group contributing to charge transport properties. The Journal of Medicinal Chemistry often features studies employing similar trifluoromethyl anilines in antidepressant drug design. Additionally, 3-methyl-2-(trifluoromethyl)aniline functions as a building block in the synthesis of agrochemical intermediates, though this application is outside the specified scope. The amine functionality enables formation of imine linkages in bioconjugation chemistry, attaching the aromatic scaffold to biomolecules for imaging purposes. Recent developments in transition metal-catalyzed coupling reactions have demonstrated the use of this aniline derivative in forming carbon-nitrogen bonds for the synthesis of complex pharmaceutical architectures.
Reviews
Write Your Own Review