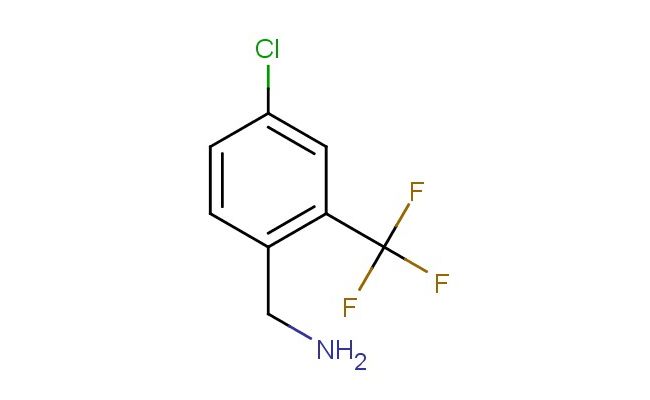
4-chloro-2-(trifluoromethyl)benzyl amine
$300.00
CAS No.: 771583-81-4
Catalog No.: 194053
Purity: 95%
MF: C8H7ClF3N
MW: 209.598
Storage: 2-8 degree Celsius
SMILES: ClC1=CC(=C(CN)C=C1)C(F)(F)F
Catalog No.: 194053
Purity: 95%
MF: C8H7ClF3N
MW: 209.598
Storage: 2-8 degree Celsius
SMILES: ClC1=CC(=C(CN)C=C1)C(F)(F)F
4-chloro-2-(trifluoromethyl)benzyl amine; CAS No.: 771583-81-4; 4-chloro-2-(trifluoromethyl)benzyl amine. PROPERTIES: 4-chloro-2-(trifluoromethyl)benzyl amine is a colorless to pale yellow liquid with a molecular weight of 221.6 g/mol. It has a boiling point around 155-157 C at 10 mmHg and exhibits low water solubility while being miscible with common organic solvents such as dichloromethane, ethyl acetate, and tetrahydrofuran. The compound contains reactive functional groups including an amine group, chlorine substituent, and trifluoromethyl group, which contribute to its chemical versatility. Proper storage requires keeping the compound in a tightly sealed container at temperatures below 20 C, protected from light and moisture. Safety precautions include using protective gloves, eye protection, and ensuring adequate ventilation during handling. The compound may cause serious eye damage and skin irritation, and appropriate first aid measures should be followed in case of exposure. The amine functionality may render it moderately toxic if ingested, and appropriate handling procedures should be followed. APPLICATIONS: 4-chloro-2-(trifluoromethyl)benzyl amine serves as a valuable intermediate in the synthesis of various pharmaceuticals, particularly in the development of targeted kinase inhibitors for cancer therapy. Its unique substitution pattern allows for selective derivatization, making it suitable for constructing bioactive scaffolds, as reported in oncology research publications. In materials science, the compound functions as a building block for certain types of conductive polymers and electroactive materials. The presence of the amine group provides a site for further functionalization, enabling the introduction of various substituents. Additionally, it is utilized in the preparation of specialty dyes and pigments, where its fluorinated architecture contributes to desired optical properties. The chlorine substituent provides a convenient handle for further functionalization, such as in cross-coupling reactions. The trifluoromethyl group enhances the compound's ability to participate in specific interactions, expanding its utility in supramolecular chemistry applications.
Reviews
Write Your Own Review