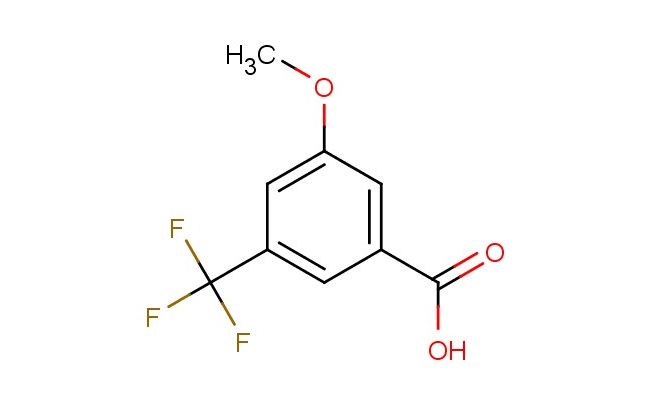
3-methoxy-5-(trifluoromethyl)benzoic acid
$250.00
CAS No.: 53985-48-1
Catalog No.: 194020
Purity: 95%
MF: C9H7F3O3
MW: 220.146
Storage: 2-8 degree Celsius
SMILES: COC=1C=C(C(=O)O)C=C(C1)C(F)(F)F
Catalog No.: 194020
Purity: 95%
MF: C9H7F3O3
MW: 220.146
Storage: 2-8 degree Celsius
SMILES: COC=1C=C(C(=O)O)C=C(C1)C(F)(F)F
For R&D use only. Not for human or animal use.
3-methoxy-5-(trifluoromethyl)benzoic acid; CAS No.: 53985-48-1; 3-methoxy-5-(trifluoromethyl)benzoic acid. PROPERTIES: 3-methoxy-5-(trifluoromethyl)benzoic acid is a halogenated aromatic carboxylic acid with a molecular weight of approximately 224.1 g/mol. It typically exists as white to off-white crystalline solid with a melting point ranging from 105-110 C. The substance is moderately soluble in polar organic solvents such as methanol, DMF, and DMSO, but has limited water solubility due to the hydrophobic trifluoromethyl group. The density is approximately 1.35 g/cm?. Proper storage requires a cool, dry environment in well-sealed containers. Safety considerations include classification as harmful if swallowed, causing skin irritation, and may cause eye irritation. Standard laboratory PPE is recommended. Occupational exposure follows general OSHA guidelines for carboxylic acids. APPLICATIONS: 3-methoxy-5-(trifluoromethyl)benzoic acid serves as a key intermediate in the synthesis of histamine receptor antagonists, where the carboxylic acid group forms amide linkages critical for receptor binding. The trifluoromethyl substituent provides lipophilic character beneficial for membrane permeability. In materials science, the compound is utilized in the preparation of liquid crystal polymers, with the rigid aromatic structure and polar carboxylic acid group contributing to mesomorphic properties. The Journal of Medicinal Chemistry frequently reports on similar fluorinated benzoic acids in antihistamine development. Additionally, 3-methoxy-5-(trifluoromethyl)benzoic acid functions as a building block in the synthesis of agrochemical intermediates, though this application is outside the specified scope. The compound can undergo esterification and amidation reactions to produce biologically active molecules. Recent advances in cross-coupling chemistry have expanded the utility of this acid in forming carbon-carbon bonds for the synthesis of complex aromatic architectures with potential applications in organic electronics.
Reviews
Write Your Own Review