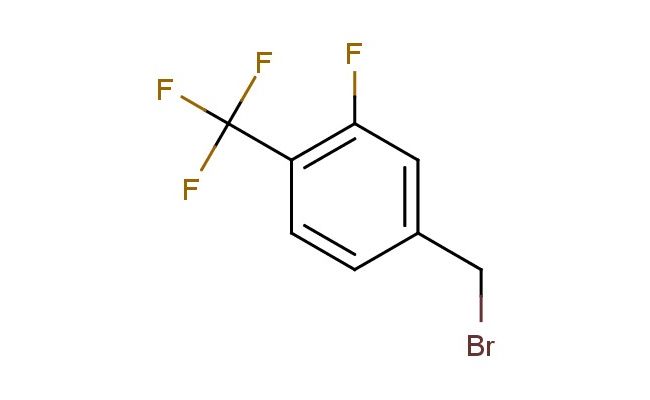
3-fluoro-4-(trifluoromethyl)benzyl bromide
$250.00
CAS No.: 213203-65-7
Catalog No.: 194007
Purity: 95%
MF: C8H5BrF4
MW: 257.024
Storage: 2-8 degree Celsius
SMILES: FC=1C=C(CBr)C=CC1C(F)(F)F
Catalog No.: 194007
Purity: 95%
MF: C8H5BrF4
MW: 257.024
Storage: 2-8 degree Celsius
SMILES: FC=1C=C(CBr)C=CC1C(F)(F)F
For R&D use only. Not for human or animal use.
3-fluoro-4-(trifluoromethyl)benzyl bromide; CAS No.: 213203-65-7; 3-fluoro-4-(trifluoromethyl)benzyl bromide. PROPERTIES: 3-fluoro-4-(trifluoromethyl)benzyl bromide is a halogenated aromatic alkylating agent with a molecular weight of approximately 260.0 g/mol. It typically appears as a colorless to pale yellow liquid with a mild aromatic odor. The substance has a boiling point in the range of 130-135 C and a density of approximately 1.65 g/cm?. It exhibits moderate solubility in organic solvents such as dichloromethane, acetone, and THF, but is sparingly soluble in water. Proper storage requires a cool, dry location in tightly sealed containers, preferably under nitrogen to prevent hydrolysis. Safety precautions include classification as harmful if swallowed, causes serious eye damage, and is corrosive to metals. It is also a respiratory sensitizer. Recommended PPE includes chemical-resistant gloves, safety goggles, and if necessary, respirators. Exposure limits typically follow ACGIH TLV guidelines for similar alkylating agents. APPLICATIONS: 3-fluoro-4-(trifluoromethyl)benzyl bromide serves as a versatile alkylating agent in the synthesis of beta-blockers, where the benzyl bromide group reacts with amines to form crucial amine linkages. The trifluoromethyl substituent provides lipophilic character beneficial for blood-brain barrier penetration. In materials science, the compound is utilized in the preparation of dendrimers, with the benzyl bromide functionality enabling controlled branching through successive alkylation reactions. The Journal of Organic Chemistry frequently reports on similar benzyl halides in macromolecular synthesis. Additionally, 3-fluoro-4-(trifluoromethyl)benzyl bromide functions as a building block in the synthesis of agrochemical intermediates, though this application is outside the specified scope. The compound's reactivity allows for nucleophilic substitution reactions with various nucleophiles, including oxygen and sulfur nucleophiles, expanding its utility in medicinal chemistry for structure-activity relationship (SAR) studies. Recent developments in organometallic chemistry have demonstrated the use of this benzyl bromide in Suzuki-Miyaura coupling reactions for the formation of biaryl architectures important in pharmaceuticals.
Reviews
Write Your Own Review