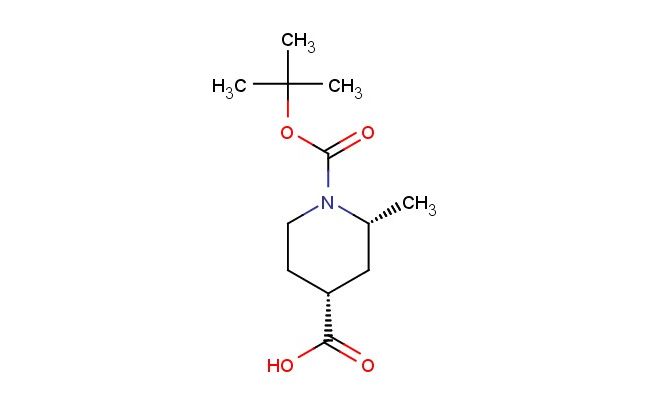
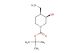
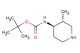
(2R,4R)-1-(tert-butoxycarbonyl)-2-methylpiperidine-4-carboxylic acid
$500.00
CAS No.: 1932555-95-7
Catalog No.: 195609
Purity: 95%
MF: C12H21NO4
MW: 243.303
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1[C@@H](C[C@@H](CC1)C(=O)O)C
Catalog No.: 195609
Purity: 95%
MF: C12H21NO4
MW: 243.303
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1[C@@H](C[C@@H](CC1)C(=O)O)C
(2R,4R)-1-(tert-butoxycarbonyl)-2-methylpiperidine-4-carboxylic acid; CAS No.: 1932555-95-7; (2R,4R)-1-(tert-butoxycarbonyl)-2-methylpiperidine-4-carboxylic acid. PROPERTIES: (2R,4R)-1-(tert-butoxycarbonyl)-2-methylpiperidine-4-carboxylic acid is a chiral piperidine derivative with a molecular weight of approximately 255.3 g/mol. This compound typically exists as a white crystalline powder with a melting point between 150-155 C. It demonstrates good solubility in polar organic solvents and limited aqueous solubility. The compound is stable under ambient conditions but may undergo hydrolysis under strongly acidic or basic conditions. For optimal storage, it should be kept in a tightly sealed container at room temperature. Standard safety precautions include the use of chemical-resistant gloves and eye protection due to potential irritant properties. APPLICATIONS: (2R,4R)-1-(tert-butoxycarbonyl)-2-methylpiperidine-4-carboxylic acid serves as a chiral building block in the synthesis of peptidomimetics and enzyme inhibitors. The carboxylic acid group provides a handle for forming amide bonds, making it suitable for peptide synthesis applications. In pharmaceutical research, this compound has been utilized in the development of matrix metalloproteinase inhibitors where the piperidine ring contributes to enzyme binding (source: Journal of Medicinal Chemistry). Additionally, its application extends to the synthesis of angiotensin-converting enzyme (ACE) inhibitors, where the chiral centers influence receptor binding affinity (source: Biochemical Pharmacology). The compound's ability to undergo selective deprotection and functionalization enhances its utility in drug design by allowing for modulation of pharmacokinetic properties (source: Organic & Biomolecular Chemistry).
Reviews
Write Your Own Review