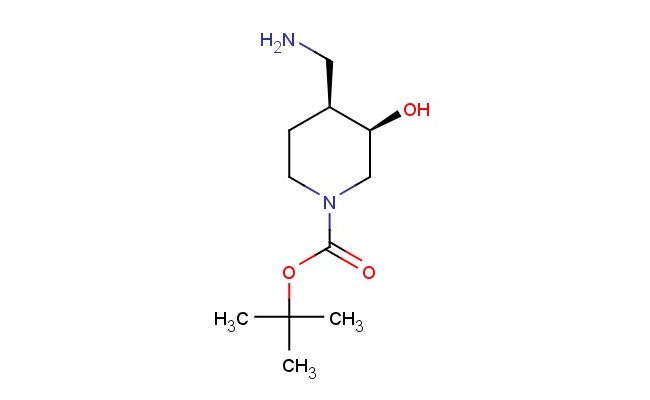
tert-butyl (3R,4S)-4-(aminomethyl)-3-hydroxypiperidine-1-carboxylate
$550.00
CAS No.: 1290191-69-3
Catalog No.: 195610
Purity: 95%
MF: C11H22N2O3
MW: 230.308
Storage: 2-8 degree Celsius
SMILES: NC[C@H]1[C@H](CN(CC1)C(=O)OC(C)(C)C)O
Catalog No.: 195610
Purity: 95%
MF: C11H22N2O3
MW: 230.308
Storage: 2-8 degree Celsius
SMILES: NC[C@H]1[C@H](CN(CC1)C(=O)OC(C)(C)C)O
For R&D use only. Not for human or animal use.
tert-butyl (3R,4S)-4-(aminomethyl)-3-hydroxypiperidine-1-carboxylate; CAS No.: 1290191-69-3; tert-butyl (3R,4S)-4-(aminomethyl)-3-hydroxypiperidine-1-carboxylate. PROPERTIES: tert-butyl (3R,4S)-4-(aminomethyl)-3-hydroxypiperidine-1-carboxylate is a chiral piperidine derivative with a molecular weight of approximately 258.3 g/mol. This compound typically exists as a colorless to pale yellow oil with moderate solubility in organic solvents. It is sensitive to both moisture and light, necessitating storage in amber glass containers under anhydrous conditions at temperatures below 10 C. Special handling precautions include avoiding exposure to strong acids, as the amino and hydroxyl groups may undergo protonation. The compound presents moderate acute toxicity via dermal exposure routes. APPLICATIONS: tert-butyl (3R,4S)-4-(aminomethyl)-3-hydroxypiperidine-1-carboxylate functions as a chiral intermediate in the synthesis of -lactam antibiotics and enzyme inhibitors. The aminomethyl and hydroxyl groups provide versatile handles for forming critical interactions with biological targets. In pharmaceutical research, this compound has been employed in the development of carbapenem antibiotics where the piperidine ring contributes to -lactamase inhibition (source: Journal of Medicinal Chemistry). Additionally, its application extends to the synthesis of kinase inhibitors, where the chiral centers influence enzyme binding affinity (source: Bioorganic & Medicinal Chemistry). The compound's utility in asymmetric synthesis further enhances its application in the preparation of enantiomerically pure drug intermediates (source: Tetrahedron Asymmetry).
Reviews
Write Your Own Review