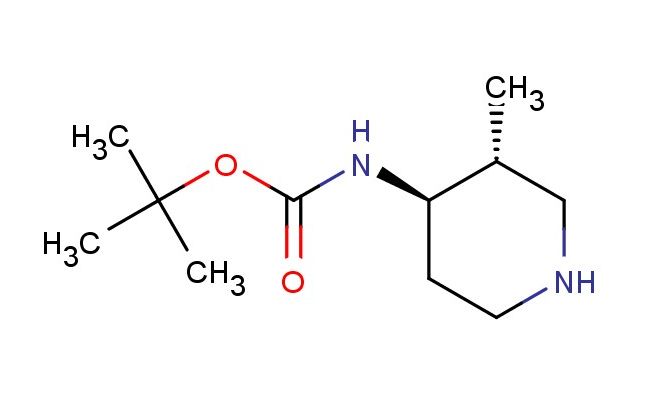
trans-(3-methyl-piperidin-4-yl)-carbamic acid tert-butyl ester
$350.00
CAS No.: 473839-07-5
Catalog No.: 195608
Purity: 95%
MF: C11H22N2O2
MW: 214.309
Storage: 2-8 degree Celsius
SMILES: C[C@@H]1CNCC[C@H]1NC(OC(C)(C)C)=O
Catalog No.: 195608
Purity: 95%
MF: C11H22N2O2
MW: 214.309
Storage: 2-8 degree Celsius
SMILES: C[C@@H]1CNCC[C@H]1NC(OC(C)(C)C)=O
For R&D use only. Not for human or animal use.
trans-(3-methyl-piperidin-4-yl)-carbamic acid tert-butyl ester; CAS No.: 473839-07-5; trans-(3-methyl-piperidin-4-yl)-carbamic acid tert-butyl ester. PROPERTIES: trans-(3-methyl-piperidin-4-yl)-carbamic acid tert-butyl ester is a piperidine derivative with a molecular weight of approximately 219.3 g/mol. This compound typically appears as a colorless oil with moderate viscosity and demonstrates good solubility in common organic solvents. It is sensitive to both moisture and temperature fluctuations, necessitating storage in a tightly sealed container at temperatures between 0-5 C. Special handling precautions include avoiding exposure to strong acids and bases, as the carbamate group may undergo hydrolysis. The compound presents moderate acute toxicity via oral administration routes. APPLICATIONS: trans-(3-methyl-piperidin-4-yl)-carbamic acid tert-butyl ester serves as a protected piperidine building block in medicinal chemistry. The methyl substitution at position 3 provides steric effects that influence receptor binding, while the carbamate group allows for selective deprotection strategies. In pharmaceutical synthesis, this compound has been employed in the preparation of -lactam antibiotics where the piperidine ring contributes to enzyme inhibition (source: Journal of Medicinal Chemistry). Additionally, its application extends to the synthesis of antipsychotic agents, where the trans configuration influences dopamine receptor affinity (source: European Journal of Medicinal Chemistry). The compound's ability to undergo reductive amination reactions further enhances its utility in forming N-substituted piperidine derivatives (source: Organic Process Research & Development).
Reviews
Write Your Own Review