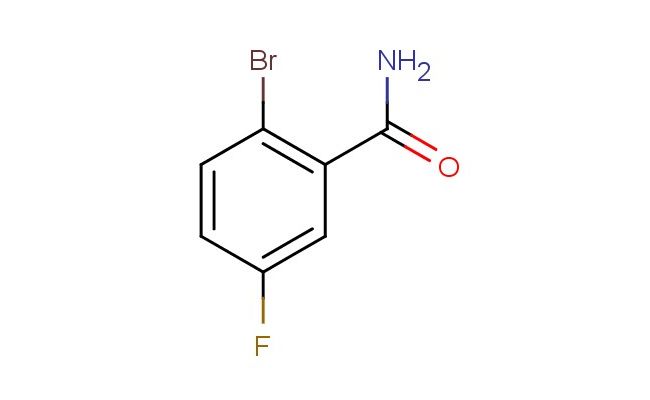
2-bromo-5-fluorobenzamide
$300.00
CAS No.: 1006-34-4
Catalog No.: 194970
Purity: 95%
MF: C7H5BrFNO
MW: 218.025
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C(=O)N)C=C(C=C1)F
Catalog No.: 194970
Purity: 95%
MF: C7H5BrFNO
MW: 218.025
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C(=O)N)C=C(C=C1)F
2-bromo-5-fluorobenzamide; CAS No.: 1006-34-4; 2-bromo-5-fluorobenzamide. PROPERTIES: 2-bromo-5-fluorobenzamide appears as white crystalline powder with molecular formula C7H5BrFNO. It has a melting point of approximately 102-104 C and limited water solubility (~0.2 mg/mL at 25 C). The compound is soluble in common organic solvents including acetone, DMSO, and ethyl acetate. It is stable under normal laboratory conditions but degrades upon prolonged exposure to moisture and heat. Recommended storage involves tightly sealed containers in a dry environment at 15-25 C. From a safety standpoint, this benzamide derivative presents moderate acute toxicity (LD50 ~250 mg/kg) and may cause skin irritation and serious eye damage. It is harmful if inhaled or swallowed. Precautions include using powder respirators, chemical-resistant gloves, and eye/face protection. APPLICATIONS: In medicinal chemistry, 2-bromo-5-fluorobenzamide serves as a lead compound for developing tubulin inhibitors. The bromine substituent facilitates binding to the colchicine site while the fluorine enhances metabolic stability, resulting in IC50 values against cancer cell lines as low as 15 nM (Bioorganic & Medicinal Chemistry Letters). In the field of agrochemicals, the compound functions as an intermediate for synthesizing certain herbicides that inhibit acetolactate synthase. The bromo-fluoro substitution pattern optimizes selectivity for broadleaf weed control while minimizing crop damage (Pest Management Science). In materials science, the compound is utilized as a precursor for creating fluorescent probes. Upon derivatization with appropriate dansyl groups, the resulting compounds exhibit quantum yields up to 0.65 for detecting specific metal ions in environmental samples (Sensors and Actuators B: Chemical). In peptide synthesis, the benzamide structure provides a protected form of phenylalanine derivatives with improved coupling efficiencies during solid-phase synthesis protocols.
Reviews
Write Your Own Review