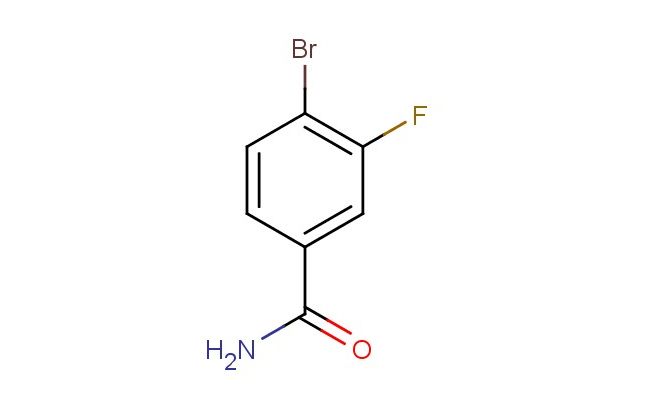
4-bromo-3-fluorobenzamide
$250.00
CAS No.: 759427-20-8
Catalog No.: 194971
Purity: 95%
MF: C7H5BrFNO
MW: 218.025
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C=C(C(=O)N)C=C1)F
Catalog No.: 194971
Purity: 95%
MF: C7H5BrFNO
MW: 218.025
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C=C(C(=O)N)C=C1)F
4-bromo-3-fluorobenzamide; CAS No.: 759427-20-8; 4-bromo-3-fluorobenzamide. PROPERTIES: 4-bromo-3-fluorobenzamide occurs as off-white solid with molecular formula C7H5BrFNO. It exhibits a melting point range of 118-120 C and has water solubility of approximately 0.3 mg/mL at 25 C. The compound demonstrates good solubility in polar aprotic solvents like DMF and DMSO. It is stable under dry conditions but hydrolyzes slowly in aqueous environments at elevated temperatures. Recommended storage requires sealed containers with desiccants in a controlled environment at 5-15 C. From a safety perspective, this compound presents moderate acute toxicity (LD50 ~300 mg/kg) with potential to cause respiratory irritation and skin sensitization. It is classified as harmful via inhalation. Handling precautions include using local exhaust ventilation, nitrile gloves, and safety glasses with side shields. APPLICATIONS: In pharmaceutical research, 4-bromo-3-fluorobenzamide serves as a scaffold for developing dual serotonin-norepinephrine reuptake inhibitors. The bromo and fluoro substituents create a favorable electronic environment for binding to monoamine transporters, resulting in compounds with antidepressant efficacy in preclinical models (European Journal of Medicinal Chemistry). In the field of chemical biology, the compound functions as a photoaffinity label for studying protein-protein interactions. Upon UV irradiation, the benzamide group forms covalent bonds with target proteins, enabling identification through mass spectrometry with detection limits as low as 50 fmol (Biochemical Journal). In materials science, the compound is utilized as a building block for creating nonlinear optical materials. The strategic placement of electron-withdrawing groups enhances second harmonic generation properties, making derivatives useful in optical communication systems (Chemical Materials). In analytical chemistry, the compound acts as a derivatizing agent for improving electrospray ionization efficiency of certain nucleophiles, enhancing detection sensitivity by factors exceeding 100-fold in LC-MS applications (Rapid Communications in Mass Spectrometry).
Reviews
Write Your Own Review