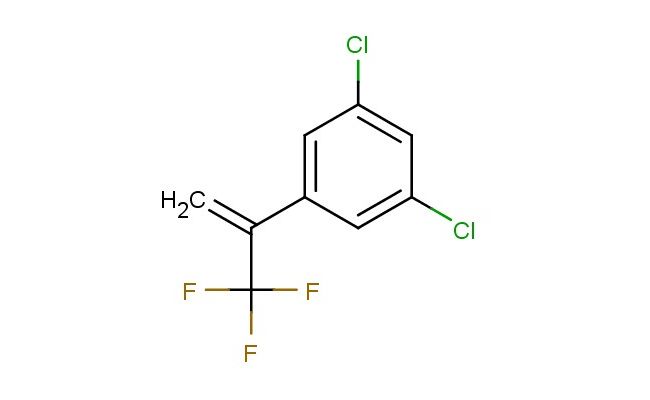
1,3-dichloro-5-(3,3,3-trifluoroprop-1-en-2-yl)benzene
$200.00
CAS No.: 864725-22-4
Catalog No.: 195028
Purity: 95%
MF: C9H5Cl2F3
MW: 241.039
Storage: 2-8 degree Celsius
SMILES: ClC1=CC(=CC(=C1)C(=C)C(F)(F)F)Cl
Catalog No.: 195028
Purity: 95%
MF: C9H5Cl2F3
MW: 241.039
Storage: 2-8 degree Celsius
SMILES: ClC1=CC(=CC(=C1)C(=C)C(F)(F)F)Cl
For R&D use only. Not for human or animal use.
1,3-dichloro-5-(3,3,3-trifluoroprop-1-en-2-yl)benzene; CAS No.: 864725-22-4; 1,3-dichloro-5-(3,3,3-trifluoroprop-1-en-2-yl)benzene. PROPERTIES: 1,3-dichloro-5-(3,3,3-trifluoroprop-1-en-2-yl)benzene is a pale yellow liquid with a slight aromatic odor. Its molecular formula C9H5Cl2F3 corresponds to a molecular weight of approximately 258.58 g/mol. The compound exhibits a boiling point around 150-155 C at 760 mmHg and a density of about 1.3 g/cm? at 20 C. It demonstrates moderate solubility in common organic solvents like dichloromethane and ethyl acetate but is sparingly soluble in water. The substance is sensitive to high temperatures and prolonged exposure to light, which may cause gradual decomposition. For proper storage, it should be kept in a tightly sealed, amber glass container in a cool, dry location away from direct sunlight and heat sources. The temperature should be maintained below 10 C if possible. Safety precautions include wearing appropriate protective equipment such as chemical-resistant gloves, safety goggles, and lab coats to prevent skin absorption and inhalation of vapors. The compound may cause skin and eye irritation, and prolonged exposure can lead to respiratory tract irritation. In case of contact, immediate rinsing with water and medical consultation is recommended. APPLICATIONS: 1,3-dichloro-5-(3,3,3-trifluoroprop-1-en-2-yl)benzene serves as a specialized intermediate in organic synthesis, particularly valuable in the preparation of fluorinated aromatic compounds used in agrochemicals and specialty chemicals. Its unique trifluoropropenyl substituent enables it to participate in nucleophilic substitution reactions and cross-coupling processes, facilitating the synthesis of complex molecular structures (Journal of Fluorine Chemistry). In materials science, the compound functions as a building block for creating liquid crystal materials with enhanced thermal stability and desirable electro-optical properties, leveraging its rigid aromatic framework and electron-withdrawing trifluoromethyl group (Liquid Crystals). Additionally, it finds application in the development of novel pharmaceutical intermediates, where its dichlorinated aromatic core provides opportunities for further functionalization and bioactive molecule design (Bioorganic & Medicinal Chemistry Letters). The compound is also employed in the synthesis of certain flame retardants and UV stabilizers, where its molecular structure contributes to effective heat absorption and light stabilization capabilities (Polymer Degradation and Stability).
Reviews
Write Your Own Review