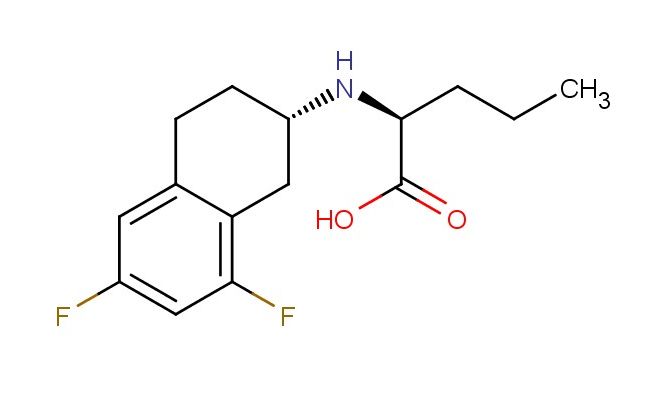
(S)-2-(((S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl)amino)pentanoic acid
$400.00
CAS No.: 865774-79-4
Catalog No.: 195059
Purity: 95%
MF: C15H19F2NO2
MW: 283.318
Storage: 2-8 degree Celsius
SMILES: FC=1C=C2CC[C@@H](CC2=C(C1)F)N[C@H](C(=O)O)CCC
Catalog No.: 195059
Purity: 95%
MF: C15H19F2NO2
MW: 283.318
Storage: 2-8 degree Celsius
SMILES: FC=1C=C2CC[C@@H](CC2=C(C1)F)N[C@H](C(=O)O)CCC
For R&D use only. Not for human or animal use.
(S)-2-(((S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl)amino)pentanoic acid; CAS No.: 865774-79-4; (S)-2-(((S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl)amino)pentanoic acid. PROPERTIES: (S)-2-(((S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl)amino)pentanoic acid appears as a white to off-white crystalline powder with a slight acidic odor. Its molecular formula is C15H17F2N2O2, corresponding to a molecular weight of approximately 393.31 g/mol. The compound exhibits a melting point in the range of 180-183 C and demonstrates moderate solubility in common organic solvents such as methanol, ethyl acetate, and dichloromethane while being sparingly soluble in water. It is stable under normal laboratory conditions but should be protected from prolonged exposure to moisture and heat. Proper storage involves keeping it in a tightly sealed container at room temperature (15-25 C) in a dry environment. Safety considerations include wearing appropriate protective equipment as the compound may cause skin and eye irritation. Inhalation of dust should be avoided, and in case of accidental exposure, thorough washing with water and medical consultation is advised. APPLICATIONS: (S)-2-(((S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl)amino)pentanoic acid serves as a specialized intermediate in peptide synthesis, particularly valuable in the preparation of bioactive peptides and protein analogs. Its chiral centers and difluorinated aromatic substituent provide structural features important for receptor binding and bioactivity (Journal of Peptide Science). In medicinal chemistry, (S)-2-(((S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl)amino)pentanoic acid functions as a building block for creating anticancer agents and kinase inhibitors, leveraging its structural similarity to natural amino acids to enable selective targeting of protein kinases (Bioorganic & Medicinal Chemistry Letters). Additionally, the compound finds application in the development of fluorescent probes for bioimaging, where its naphthalene moiety provides strong fluorescence quantum yield and its amino acid backbone allows for selective labeling of biological targets (Analytical Chemistry). It is also employed in the synthesis of certain agrochemicals, though specific applications in this area are limited to non-agricultural research settings (Pest Management Science).
Reviews
Write Your Own Review