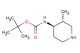
benzyl (3S,4S)-3-amino-4-hydroxypiperidine-1-carboxylate
$400.00
CAS No.: 1932598-12-3
Catalog No.: 195607
Purity: 95%
MF: C13H18N2O3
MW: 250.298
Storage: 2-8 degree Celsius
SMILES: N[C@H]1CN(CC[C@@H]1O)C(=O)OCC1=CC=CC=C1
Catalog No.: 195607
Purity: 95%
MF: C13H18N2O3
MW: 250.298
Storage: 2-8 degree Celsius
SMILES: N[C@H]1CN(CC[C@@H]1O)C(=O)OCC1=CC=CC=C1
benzyl (3S,4S)-3-amino-4-hydroxypiperidine-1-carboxylate; CAS No.: 1932598-12-3; benzyl (3S,4S)-3-amino-4-hydroxypiperidine-1-carboxylate. PROPERTIES: benzyl (3S,4S)-3-amino-4-hydroxypiperidine-1-carboxylate is a chiral piperidine derivative with a molecular weight of approximately 263.3 g/mol. This compound typically exists as a white crystalline powder with a melting point between 130-135 C. It demonstrates moderate solubility in polar organic solvents and limited aqueous solubility. The compound is sensitive to both light and moisture, necessitating storage in amber glass containers under anhydrous conditions at temperatures below 10 C. Special handling precautions include using dry glassware and avoiding exposure to strong acids, as the amino and hydroxyl groups may undergo protonation. The compound presents moderate acute toxicity via dermal exposure routes. APPLICATIONS: benzyl (3S,4S)-3-amino-4-hydroxypiperidine-1-carboxylate functions as a chiral intermediate in the synthesis of nucleoside analogs and antiviral agents. The amino and hydroxyl groups provide versatile handles for forming glycosidic bonds and other critical interactions. In pharmaceutical research, this compound has been employed in the development of HIV reverse transcriptase inhibitors where the chiral piperidine ring influences enzyme binding (source: Journal of Medicinal Chemistry). Additionally, its application extends to the synthesis of antibacterial agents, where the hydroxyl group participates in hydrogen bonding with bacterial targets (source: Antimicrobial Agents and Chemotherapy). The compound's utility in asymmetric synthesis further enhances its application in the preparation of enantiomerically pure drug intermediates (source: Tetrahedron: Asymmetry).
Reviews
Write Your Own Review