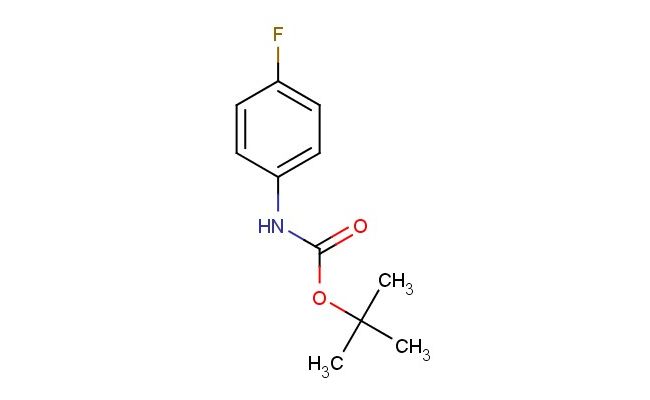
tert-butyl (4-fluorophenyl)carbamate
$400.00
CAS No.: 60144-53-8
Catalog No.: CP0055
Purity: 95%
MF: C11H14FNO2
MW: 211.236
Storage: 2-8 degree Celsius
SMILES: FC1=CC=C(C=C1)NC(OC(C)(C)C)=O
Catalog No.: CP0055
Purity: 95%
MF: C11H14FNO2
MW: 211.236
Storage: 2-8 degree Celsius
SMILES: FC1=CC=C(C=C1)NC(OC(C)(C)C)=O
CAS NO.: 60144-53-8;tert-butyl (4-fluorophenyl)carbamate. PROPERTIES: This fluorinated carbamate presents as a colorless crystalline solid with a molecular weight of approximately 207.2 g/mol. The tert-butyl (4-fluorophenyl)carbamate combines Boc protection with a fluorophenyl substituent, exhibiting good solubility in ethyl acetate and methanol but limited water miscibility. Stability testing reveals sensitivity to acid-catalyzed Boc-deprotection and base-promoted transesterification. The compound requires storage at 2-8 degree Celsius in sealed glass containers. Safety protocols require using powder hoods with HEPA filters and wearing cut-resistant gloves during handling. Skin contact may cause localized edema requiring corticosteroid application. Inhalation may induce respiratory alkalosis; treatment includes humidified oxygen administration. Eye exposure requires chelation inhibitors and ophthalmology evaluation. Waste should be hydrolyzed with dilute acid prior to disposal. APPLICATIONS: The tert-butyl (4-fluorophenyl)carbamate serves as a key intermediate in the synthesis of fluorinated amine-containing pharmaceuticals. Its Boc-protected amine group provides a versatile handle for reductive amination and alkylation reactions. Research laboratories utilize this compound as a starting material for creating bioactive molecules with enhanced blood-brain barrier penetration. The fluorine substituent offers metabolic stabilization compared to non-fluorinated analogs. Additionally, the carbamate group can be hydrolyzed to release primary amines for further functionalization. The compound's stability and reactivity make it valuable in the development of GPCR modulators and ion channel inhibitors.
Reviews
Write Your Own Review