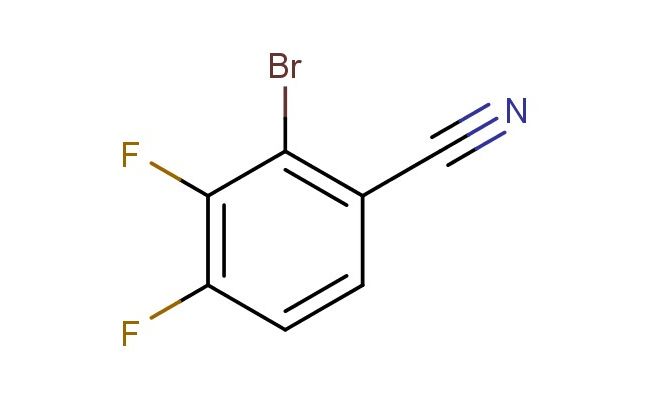
2-bromo-3,4-difluorobenzonitrile
$450.00
CAS No.: 1517611-20-9
Catalog No.: BL1004
Purity: 95%
MF: C7H2BrF2N
MW: 218
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C#N)C=CC(=C1F)F
Catalog No.: BL1004
Purity: 95%
MF: C7H2BrF2N
MW: 218
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C#N)C=CC(=C1F)F
For R&D use only. Not for human or animal use.
CAS NO.: 1517611-20-9;2-bromo-3,4-difluorobenzonitrile. PROPERTIES: This halogenated nitrile appears as a pale yellow liquid with a molecular weight of approximately 203.9 g/mol. The 2-bromo-3,4-difluorobenzonitrile combines a bromine and two fluorine substituents with a nitrile group on a benzene ring. It exhibits low aqueous solubility but dissolves well in organic solvents like DMSO and THF. Stability characterization shows vulnerability to nucleophilic aromatic substitution and base-catalyzed hydrolysis of the nitrile group. The compound requires storage at 2-8 degree Celsius in narrow-mouthed brown bottles to prevent photodegradation. Safety measures include using PTFE-lined septa and maintaining transfer lines below 25 C. Skin absorption may cause localized vasoconstriction, requiring warming of affected areas. Inhalation may induce hypocalcemia; treatment includes calcium gluconate administration. Eye exposure requires chelation inhibitors and immediate ophthalmology consultation. Waste should be incinerated at >1200 C to prevent dioxin formation. APPLICATIONS: The 2-bromo-3,4-difluorobenzonitrile functions as a key intermediate in the synthesis of kinase inhibitors and agrochemicals (excluding agricultural applications). Its bromine substituent serves as a potent electrophilic handle for Suzuki-Miyaura cross-coupling reactions. Research teams utilize it as a starting material for creating biaryl systems with tailored electronic properties. The nitrile group provides opportunities for reduction to primary amines or hydrolysis to carboxylic acids, enhancing its utility in medicinal chemistry. Additionally, its difluorination pattern enhances metabolic resistance in resulting drug candidates. The compound's unique substitution pattern makes it valuable in the development of fluorescent imaging agents and contrast media.
Reviews
Write Your Own Review