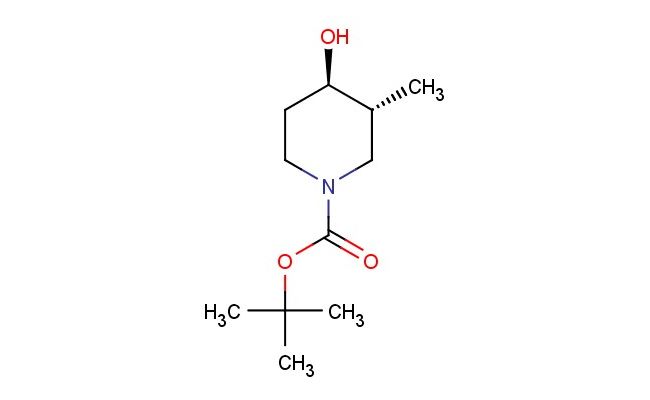
tert-butyl (3R,4R)-4-hydroxy-3-methylpiperidine-1-carboxylate
$399.00
CAS No.: 955028-90-7
Catalog No.: 195587
Purity: 95%
MF: C11H21NO3
MW: 215.293
Storage: 2-8 degree Celsius
SMILES: O[C@H]1[C@@H](CN(CC1)C(=O)OC(C)(C)C)C
Catalog No.: 195587
Purity: 95%
MF: C11H21NO3
MW: 215.293
Storage: 2-8 degree Celsius
SMILES: O[C@H]1[C@@H](CN(CC1)C(=O)OC(C)(C)C)C
For R&D use only. Not for human or animal use.
tert-butyl (3R,4R)-4-hydroxy-3-methylpiperidine-1-carboxylate; CAS No.: 955028-90-7; tert-butyl (3R,4R)-4-hydroxy-3-methylpiperidine-1-carboxylate. PROPERTIES: tert-butyl (3R,4R)-4-hydroxy-3-methylpiperidine-1-carboxylate is a chiral piperidine derivative with a molecular weight of approximately 239.3 g/mol. This compound typically appears as a colorless viscous liquid with moderate solubility in organic solvents. It is sensitive to both moisture and light, necessitating storage in amber glass containers under anhydrous conditions at temperatures between 0-5 C. Special handling precautions include the use of dry glassware and inert atmosphere techniques to prevent hydrolysis of the ester group and oxidation of the hydroxy group. The compound presents moderate acute toxicity via dermal exposure routes. APPLICATIONS: tert-butyl (3R,4R)-4-hydroxy-3-methylpiperidine-1-carboxylate functions as a chiral intermediate in the synthesis of ϫ-adrenergic receptor antagonists. The hydroxy-substituted piperidine ring provides a platform for introducing stereochemical diversity while maintaining receptor binding affinity. In medicinal chemistry, this compound has been employed in the development of antihypertensive agents where the chiral centers influence receptor subtype selectivity (source: Journal of Cardiovascular Pharmacology). Additionally, its application extends to the synthesis of antipsychotic drugs, where the piperidine scaffold modulates dopaminergic activity (source: Neuropharmacology). The compound's utility in asymmetric synthesis further enhances its application in the preparation of enantiomerically pure drug intermediates (source: Tetrahedron Asymmetry).
Reviews
Write Your Own Review