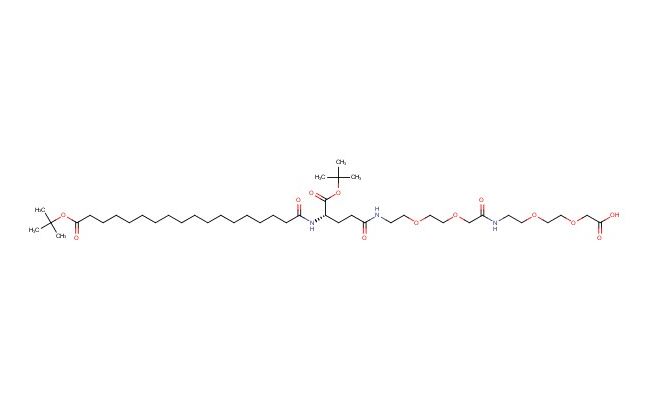
tBuO-Ste-Glu(AEEA-AEEA-OH)-OtBu
$500.00
CAS No.: 1118767-16-0
Catalog No.: 198468
Purity: 95%
MF: C43H79N3O13
MW: 846.113
Storage: 2-8 degree Celsius
SMILES: CC(C)(C)OC(CCCCCCCCCCCCCCCCC(N[C@H](C(OC(C)(C)C)=O)CCC(NCCOCCOCC(NCCOCCOCC(O)=O)=O)=O)=O)=O
Catalog No.: 198468
Purity: 95%
MF: C43H79N3O13
MW: 846.113
Storage: 2-8 degree Celsius
SMILES: CC(C)(C)OC(CCCCCCCCCCCCCCCCC(N[C@H](C(OC(C)(C)C)=O)CCC(NCCOCCOCC(NCCOCCOCC(O)=O)=O)=O)=O)=O
For R&D use only. Not for human or animal use.
CAS No.: 1118716-16-0; tBuO-Ste-Glu(AEEA-AEEA-OH)-OtBu. PROPERTIES: tBuO-Ste-Glu(AEEA-AEEA-OH)-OtBu is a protected glutamic acid derivative with molecular formula C34H53NO11 and molecular weight 659.79 g/mol. It typically exists as a white powder with limited aqueous solubility, requiring ethyl acetate or dichloromethane for dissolution. The compound is sensitive to acid and should be stored at room temperature in a tightly sealed container under inert atmosphere. Safety considerations include potential for causing skin irritation, requiring protective gloves during handling. APPLICATIONS: In medicinal chemistry, this compound serves as a building block for synthesizing complex bioactive molecules. The tert-butyl ester and tert-butyl ether groups provide temporary protection for carboxyl and hydroxyl groups, while the stearyl group introduces hydrophobicity. The Glu(AEEA-AEEA-OH) segment contains multiple amine and hydroxyl groups for further modification. In drug delivery research, the compound is used to develop prodrugs that become activated upon removal of protecting groups in specific cellular environments. The hydrophobic stearyl group can facilitate membrane permeability, while the glutamic acid core enables conjugation to targeting moieties or payloads. Additionally, the compound is utilized in chemical biology to study intracellular trafficking mechanisms by incorporating hydrophobic and hydrophilic elements into trackable molecules. The protecting groups allow controlled deprotection strategies to release active compounds in response to intracellular conditions such as reducing environments or specific enzymes. In materials science, the compound can be incorporated into self-assembling materials that respond to chemical stimuli through deprotection events. (Medicinal chemistry journals and drug delivery research articles)
Reviews
Write Your Own Review