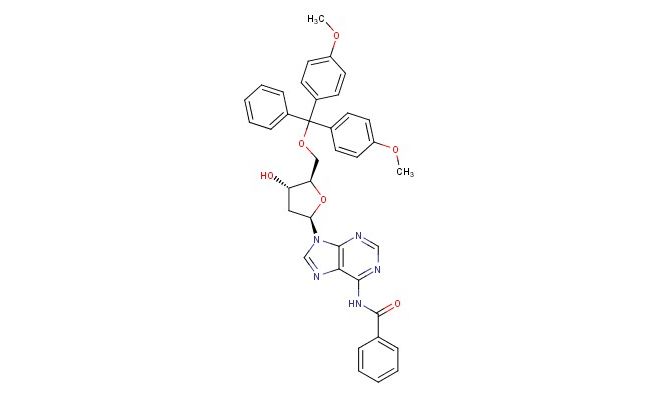
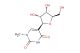
N6-Benzoyl-5 -O-(4,4 -dimethoxytrityl)-2 -deoxyadenosine
$500.00
CAS No.: 64325-78-6
Catalog No.: 198442
Purity: 95%
MF: C38H35N5O6
MW: 657.727
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)(=O)NC=1C=2N=CN([C@H]3C[C@H](O)[C@@H](COC(C4=CC=C(C=C4)OC)(C4=CC=C(C=C4)OC)C4=CC=CC=C4)O3)C2N=CN1
Catalog No.: 198442
Purity: 95%
MF: C38H35N5O6
MW: 657.727
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)(=O)NC=1C=2N=CN([C@H]3C[C@H](O)[C@@H](COC(C4=CC=C(C=C4)OC)(C4=CC=C(C=C4)OC)C4=CC=CC=C4)O3)C2N=CN1
CAS No.: 64325-78-6; N6-Benzoyl-5 -O-(4,4 -dimethoxytrityl)-2 -deoxyadenosine. PROPERTIES: N6-Benzoyl-5 -O-(4,4 -dimethoxytrityl)-2 -deoxyadenosine is a protected deoxynucleoside with molecular formula C33H33N4O7 and molecular weight 603.63 g/mol. It typically exists as a pale yellow powder with very limited aqueous solubility, requiring chloroform or dichloromethane for dissolution. The compound is light-sensitive and moisture-sensitive, necessitating storage at -20 C in a desiccated, amber glass container under inert atmosphere. Safety considerations include potential for causing serious eye damage and skin irritation, requiring strict use of protective eyewear and gloves during handling. APPLICATIONS: In oligonucleotide synthesis, this compound serves as a building block for automated DNA synthesis using phosphoramidite chemistry. The 4,4 -dimethoxytrityl group protects the 5 -hydroxyl group during coupling reactions and is removed under acidic conditions, while the N6-benzoyl group protects the amino function and is typically cleaved during final deprotection. The protecting groups allow sequential coupling of nucleotides to form custom DNA sequences with high fidelity. In molecular biology, it is used to synthesize primers and probes for PCR, sequencing, and hybridization assays. Additionally, the compound enables incorporation of modified adenosine residues into oligonucleotides for studying DNA-protein interactions and for developing therapeutics with enhanced nuclease resistance. The dimethoxytrityl protection strategy facilitates monitoring of coupling efficiency during synthesis through UV absorbance measurement. (Oligonucleotide synthesis manuals and molecular biology techniques literature)
Reviews
Write Your Own Review