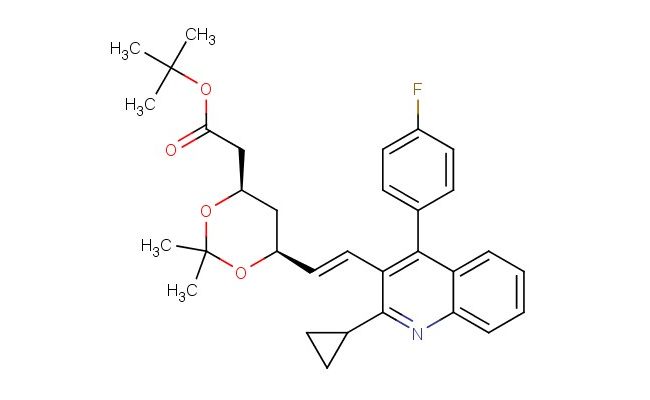
(4R,6S)-6-[(1E)-2-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester
$350.00
CAS No.: 147489-06-3
Catalog No.: 109964
Purity: 95%
MF: C32H36FNO4
MW: 517.641
Storage: 2-8 degree Celsius
SMILES: CC(C)(C)OC(=O)C[C@H]1C[C@H](OC(C)(C)O1)\C=C\C1=C(C2=CC=C(F)C=C2)C2=CC=CC=C2N=C1C1CC1
Catalog No.: 109964
Purity: 95%
MF: C32H36FNO4
MW: 517.641
Storage: 2-8 degree Celsius
SMILES: CC(C)(C)OC(=O)C[C@H]1C[C@H](OC(C)(C)O1)\C=C\C1=C(C2=CC=C(F)C=C2)C2=CC=CC=C2N=C1C1CC1
For R&D use only. Not for human or animal use.
(4R,6S)-6-[(1E)-2-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester; CAS No.: 147489-06-3; This dienyl dioxane acetate showcases ChemShuttle s key intermediates for conjugated system statins. The (4R,6S) configuration and tert-butyl ester facilitate downstream hydrolysis to active acids. Our Heck coupling process maintains stereochemical integrity during vinyl group installation. The product enables efficient synthesis of pravastatin analogs with modified hydrophobic domains.
Reviews
Write Your Own Review




![tert-butyl (4R,6R)-2-[[[6-(2-4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/0/109978_2.jpg)