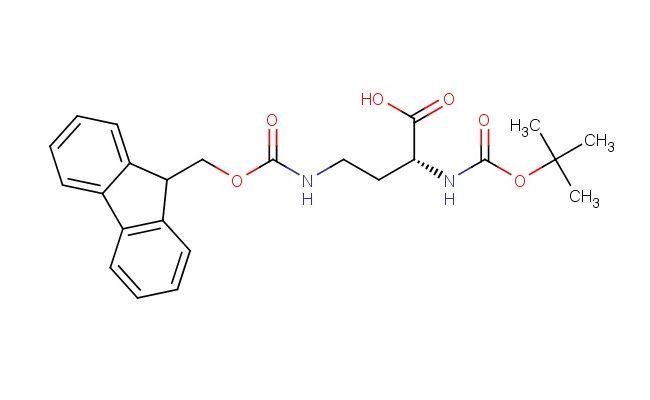
(R)-4-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-2-((tert-butoxycarbonyl)amino)butanoic acid
$200.00
CAS No.: 131570-57-5
Catalog No.: WLZ0976
Purity: 95%
MF: C24H28N2O6
MW: 440.496
Storage: 2-8 degree Celsius
SMILES: C1=CC=CC=2C3=CC=CC=C3C(C12)COC(=O)NCC[C@H](C(=O)O)NC(=O)OC(C)(C)C
Catalog No.: WLZ0976
Purity: 95%
MF: C24H28N2O6
MW: 440.496
Storage: 2-8 degree Celsius
SMILES: C1=CC=CC=2C3=CC=CC=C3C(C12)COC(=O)NCC[C@H](C(=O)O)NC(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
CAS NO.: 131570-57-5; (R)-4-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-2-((tert-butoxycarbonyl)amino)butanoic acid. PROPERTIES: This compound is a sophisticated organic molecule featuring dual protective groups, with a fluorenylmethoxycarbonyl (Fmoc) moiety on the primary amine and a tert-butoxycarbonyl (Boc) group on the secondary amine. The (R)-4-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-2-((tert-butoxycarbonyl)amino)butanoic acid typically presents as a white to off-white crystalline solid with limited aqueous solubility but good solubility in common organic solvents like dimethylformamide (DMF) and dichloromethane. Its molecular structure includes a chiral center at the -carbon, which is critical for maintaining stereochemical integrity during synthetic applications. For optimal stability and to prevent premature deprotection, the compound should be stored at 2-8 degree Celsius in a tightly sealed container away from moisture and direct light. When handling this material, appropriate personal protective equipment including nitrile gloves, safety goggles, and a lab coat is recommended. Work areas should be equipped with proper ventilation to minimize inhalation risks. In case of skin contact, wash thoroughly with soap and water; if eye contact occurs, rinse immediately with plenty of water and seek medical advice. APPLICATIONS: The (R)-4-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-2-((tert-butoxycarbonyl)amino)butanoic acid serves as a valuable building block in peptide synthesis, particularly for creating complex peptide architectures where orthogonal protection strategies are essential. Its dual protection allows for sequential deprotection and coupling steps, enabling precise control over peptide assembly. In medicinal chemistry research, this compound facilitates the synthesis of bioactive peptides with defined stereochemistry, which is crucial for developing therapeutic agents targeting various disease pathways. Additionally, the molecule finds utility in chemical biology studies where specific amino acid derivatives are required to probe protein function and structure. The orthogonal protection strategy of this compound also makes it suitable for solid-phase peptide synthesis (SPPS) workflows, where efficient resin attachment and stepwise elongation depend on reliable protecting group chemistries. Researchers utilizing this compound can leverage its well-defined stereochemistry and protection profile to advance investigations into peptide-based drug discovery and development.
Reviews
Write Your Own Review