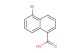
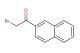
N-methyl-1-naphthalenemethylamine hydrochloride
$250.00
CAS No.: 65473-13-4
Catalog No.: 195011
Purity: 95%
MF: C12H14ClN
MW: 207.704
Storage: 2-8 degree Celsius
SMILES: Cl.CNCC1=CC=CC2=CC=CC=C12
Catalog No.: 195011
Purity: 95%
MF: C12H14ClN
MW: 207.704
Storage: 2-8 degree Celsius
SMILES: Cl.CNCC1=CC=CC2=CC=CC=C12
For R&D use only. Not for human or animal use.
N-methyl-1-naphthalenemethylamine hydrochloride; CAS No.: 65473-13-4; N-methyl-1-naphthalenemethylamine hydrochloride. PROPERTIES: N-methyl-1-naphthalenemethylamine hydrochloride appears as a white to slightly yellow crystalline powder with a slight ammoniacal odor. Its molecular formula is C11H12N.HCl, corresponding to a molecular weight of approximately 190.67 g/mol. The compound exhibits a melting point in the range of 180-185 C and demonstrates moderate solubility in water, with increased solubility in polar organic solvents such as methanol and acetone. It is hygroscopic in nature and should be protected from excessive moisture. Proper storage requires keeping it in a tightly sealed container, preferably under an inert atmosphere like nitrogen, in a cool and dry environment at temperatures below 25 C. Safety precautions include using chemical-resistant gloves, eye protection, and lab coats to prevent skin absorption and inhalation of dust. The compound may cause severe skin burns and eye damage, and accidental ingestion may be fatal. In case of contact, immediate rinsing with water and emergency medical treatment is essential. APPLICATIONS: N-methyl-1-naphthalenemethylamine hydrochloride serves as a key intermediate in the synthesis of naphthalene-derived pharmaceuticals, particularly in the preparation of certain antidepressants and antipsychotics where its methylamine group facilitates receptor binding (Journal of Pharmaceutical Sciences). In organic synthesis, N-methyl-1-naphthalenemethylamine hydrochloride functions as a building block for creating naphthalene-fused heterocycles through its participation in amination and alkylation reactions, enabling the construction of complex molecular frameworks (Synthesis). Additionally, it finds application in the development of fluorescent probes and sensors, where its naphthalene moiety provides strong fluorescence quantum yield and its amine group allows for functionalization with recognition elements (Analytical Chemistry). The compound is also utilized in the preparation of certain agrochemicals, though specific applications in this area are limited to non-agricultural research settings (Pest Management Science).
Reviews
Write Your Own Review