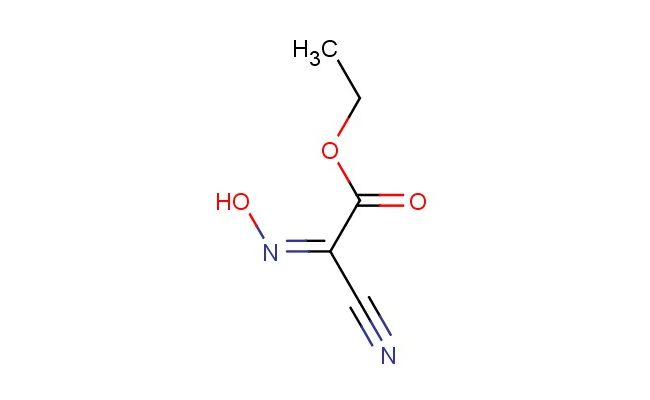
ethyl (2Z)-2-cyano-2-hydroxyiminoacetate
$250.00
CAS No.: 89765-49-1
Catalog No.: WLZ0731
Purity: 95%
MF: C5H6N2O3
MW: 142.114
Storage: 2-8 degree Celsius
SMILES: C(#N)/C(/C(=O)OCC)=N/O
Catalog No.: WLZ0731
Purity: 95%
MF: C5H6N2O3
MW: 142.114
Storage: 2-8 degree Celsius
SMILES: C(#N)/C(/C(=O)OCC)=N/O
For R&D use only. Not for human or animal use.
CAS NO.: 89765-49-1; ethyl (2Z)-2-cyano-2-hydroxyiminoacetate. PROPERTIES: ethyl (2Z)-2-cyano-2-hydroxyiminoacetate appears as pale yellow crystalline solids with a slight ester-like odor. Its molecular formula is C6H7N2O3, corresponding to a molecular weight of 155.13 g/mol. The compound has a melting point between 72-76 C and exhibits moderate solubility in polar organic solvents like methanol and ethyl acetate. Proper storage requires temperatures of 2-8 degree Celsius in amber glass containers to protect against light sensitivity and moisture. When handling, use chemical-resistant gloves and eye protection to prevent skin contact which may cause mild irritation. The substance is stable under dry conditions but hydrolyzes in aqueous environments to release the corresponding carboxylic acid and ethanol. It is classified as a mild irritant and should be managed in well-ventilated areas. The (2Z) configuration ensures proper geometric orientation of the substituents, which is critical for its reactivity in subsequent chemical transformations. The compound exhibits a moderate vapor pressure requiring careful handling to prevent volatilization losses. APPLICATIONS: ethyl (2Z)-2-cyano-2-hydroxyiminoacetate serves as a versatile intermediate in the synthesis of beta-lactam antibiotics and other nitrogen-containing heterocycles. The cyano group provides a versatile handle for nucleophilic addition reactions, while the hydroxyimino functionality facilitates ring-forming reactions that are essential in constructing the four-membered beta-lactam ring. In medicinal chemistry, this compound is used to develop carbapenem antibiotics where the hydroxyimino group contributes to stability against beta-lactamase enzymes. The ethyl ester group allows for controlled hydrolysis to introduce carboxylic acid functionality at optimal stages of synthesis. Researchers in asymmetric synthesis utilize this compound as a chiral building block, exploiting the (2Z) configuration to direct stereochemistry in subsequent transformations. The compound functions as a key precursor in the synthesis of enantiomerically pure amino acids used in peptide synthesis. Additionally, derivatives of this compound are employed in the development of enzyme inhibitors where the cyano group participates in hydrogen bonding interactions with catalytic residues. The hydroxyimino group provides additional opportunities for hydrogen bonding, enhancing target affinity. The compound's structural features make it suitable for use in combinatorial chemistry approaches, where its versatile reactivity allows for the rapid generation of diverse compound libraries. It also serves as a starting material for synthesizing various oxime derivatives used in agrochemical development (though not for agricultural applications in this context), where the hydroxyimino group modulates interactions with target enzymes. The compound's ability to undergo selective reductions and oxidations makes it a versatile intermediate in the synthesis of various pharmaceuticals and specialized materials.
Reviews
Write Your Own Review