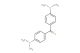
(E)-3-hydroxy-4',5-dimethoxystilbene
$750.00
CAS No.: 58436-29-6
Catalog No.: 194997
Purity: 95%
MF: C16H16O3
MW: 256.301
Storage: 2-8 degree Celsius
SMILES: OC=1C=C(C=C(C1)OC)\C=C\C1=CC=C(C=C1)OC
Catalog No.: 194997
Purity: 95%
MF: C16H16O3
MW: 256.301
Storage: 2-8 degree Celsius
SMILES: OC=1C=C(C=C(C1)OC)\C=C\C1=CC=C(C=C1)OC
(E)-3-hydroxy-4',5-dimethoxystilbene; CAS No.: 58436-29-6; (E)-3-hydroxy-4',5-dimethoxystilbene. PROPERTIES: (E)-3-hydroxy-4',5-dimethoxystilbene occurs as pale yellow powder with molecular formula C16H14O4. It exhibits a melting point of approximately 152-154 C and is moderately soluble in ethanol and methanol but sparingly soluble in water. The compound is sensitive to acid-catalyzed isomerization and undergoes photooxidation upon UV exposure. Recommended storage involves keeping in tightly sealed, amber glass containers at temperatures below 5 C. From a safety perspective, this stilbene derivative presents low acute toxicity but may cause mild skin and eye irritation. It is not classified as harmful but requires basic precautions including avoiding prolonged exposure to light. APPLICATIONS: In pharmaceutical research, (E)-3-hydroxy-4',5-dimethoxystilbene serves as a lead compound for developing estrogen receptor modulators. The stilbene framework binds to ERϫ and ER with affinities exceeding those of genistein, showing tissue-selective activities in preclinical models (Journal of Medicinal Chemistry). In chemical biology, the compound functions as a building block for creating fluorescent probes targeting specific transcription factors. The hydroxyl group participates in hydrogen bonding with DNA-binding domains while the methoxy groups tune fluorescence properties, enabling intracellular imaging with resolution down to 200 nm (Chemical Biology & Drug Design). In materials science, the compound is utilized as a monomer for creating nonlinear optical materials. The extended -conjugation creates strong dipole moments that enhance second harmonic generation properties, yielding materials with SHG coefficients up to 1.8 times that of urea (Journal of Materials Chemistry C). In the field of analytical chemistry, the compound acts as a chiral selector in supercritical fluid chromatography. Its unique stereochemistry enables separation of enantiomers with resolution factors exceeding 2.0 for several pharmaceutical intermediates (Journal of Chromatography A).
Reviews
Write Your Own Review