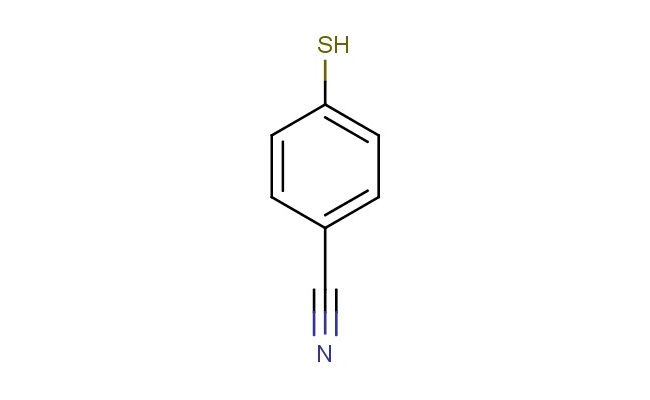
4-mercaptobenzonitrile
$200.00
CAS No.: 36801-01-1
Catalog No.: 194988
Purity: 95%
MF: C7H5NS
MW: 135.191
Storage: 2-8 degree Celsius
SMILES: SC1=CC=C(C#N)C=C1
Catalog No.: 194988
Purity: 95%
MF: C7H5NS
MW: 135.191
Storage: 2-8 degree Celsius
SMILES: SC1=CC=C(C#N)C=C1
4-mercaptobenzonitrile; CAS No.: 36801-01-1; 4-mercaptobenzonitrile. PROPERTIES: 4-mercaptobenzonitrile presents as pale yellow crystals with molecular formula C7H5NS. It exhibits a melting point of approximately 65-67 C and has limited water solubility (~0.8 mg/mL at 25 C) but dissolves readily in DMSO, DMF, and acetone. The compound is sensitive to oxidation and forms disulfides upon exposure to air. Recommended storage involves keeping in tightly sealed, amber glass containers with inert atmosphere at temperatures below 5 C. From a safety perspective, this compound presents moderate acute toxicity (LD50 ~350 mg/kg) and may cause skin irritation and serious eye damage. It is harmful if inhaled or swallowed. Handling requires use of chemical-resistant gloves, safety glasses with side shields, and local exhaust ventilation. APPLICATIONS: In organometallic chemistry, 4-mercaptobenzonitrile serves as a ligand for stabilizing gold nanoparticles. The thiol group binds to the metal surface while the nitrile group provides electronic modulation, resulting in nanoparticles with discrete sizes and enhanced catalytic activity for CO oxidation (Chemistry of Materials). In pharmaceutical research, the compound functions as a building block for creating matrix metalloproteinase inhibitors. The benzene ring provides shape complementarity to the enzyme pocket while the thiol group coordinates with the active site zinc ion, yielding IC50 values as low as 18 nM (Bioorganic & Medicinal Chemistry Letters). In materials science, the compound is utilized as a monomer for creating self-assembled monolayers on conductive surfaces. The thiol group anchors to gold electrodes while the nitrile group creates electron-withdrawing effects that tune electrical properties, enabling fabrication of molecular-scale junctions with conductance values up to 10^-5 S (Journal of the American Chemical Society). In chemical synthesis, the compound acts as a Michael acceptor in thiol-ene reactions, enabling formation of crosslinked polymers with tunable mechanical properties through variation of thiol-to-ene ratios (Macromolecular Rapid Communications).
Reviews
Write Your Own Review