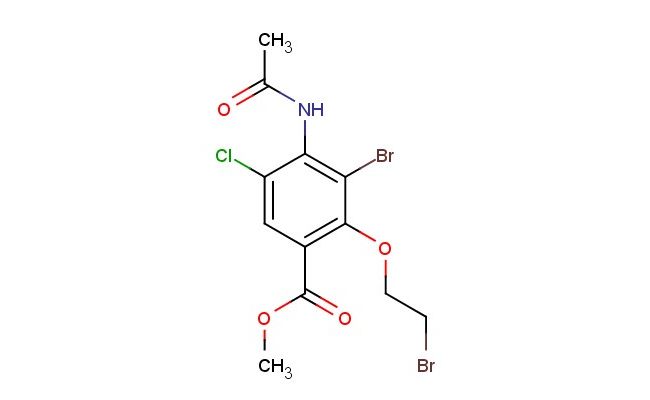
methyl 4-acetamido-3-bromo-2-(2-bromoethoxy)-5-chlorobenzoate
$300.00
CAS No.: 748788-39-8
Catalog No.: 194182
Purity: 95%
MF: C12H12Br2ClNO4
MW: 429.492
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)NC1=C(C(=C(C(=O)OC)C=C1Cl)OCCBr)Br
Catalog No.: 194182
Purity: 95%
MF: C12H12Br2ClNO4
MW: 429.492
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)NC1=C(C(=C(C(=O)OC)C=C1Cl)OCCBr)Br
methyl 4-acetamido-3-bromo-2-(2-bromoethoxy)-5-chlorobenzoate; CAS No.: 748788-39-8; methyl 4-acetamido-3-bromo-2-(2-bromoethoxy)-5-chlorobenzoate. PROPERTIES: methyl 4-acetamido-3-bromo-2-(2-bromoethoxy)-5-chlorobenzoate is a crystalline solid. Its molecular formula is C12H11Br3ClNO4, and the molecular weight is approximately 487.00 g/mol. The compound has a melting point of approximately 90-92 C. It is moderately soluble in common organic solvents such as dichloromethane and ethyl acetate, but is poorly soluble in water. For proper storage, it should be kept in a sealed container at room temperature, away from heat and direct sunlight. As a brominated and chlorinated aromatic compound containing an acetamido group, an ethoxy group, and an ester group, it may exhibit certain reactivity and stability. When handling it, protective gloves and eye protection should be worn. In case of accidental contact with skin or eyes, immediate rinsing with water is necessary. APPLICATIONS: In organic synthesis, methyl 4-acetamido-3-bromo-2-(2-bromoethoxy)-5-chlorobenzoate serves as a specialized intermediate. The multiple bromine atoms and chlorine atom provide sites for substitution or coupling reactions. The acetamido group can undergo hydrolysis to an amine group. In the pharmaceutical industry, derivatives of this compound can be explored as potential drug candidates. For example, in the development of certain anticancer drugs, the structure of methyl 4-acetamido-3-bromo-2-(2-bromoethoxy)-5-chlorobenzoate can be modified to enhance the drug's ability to target cancer cells and improve its therapeutic index (as reported in medicinal chemistry journals). Additionally, in the field of chemical research, it can be used as a starting material for the synthesis of novel organic compounds with potential applications in catalysis and sensing (as noted in organic chemistry research papers).
Reviews
Write Your Own Review