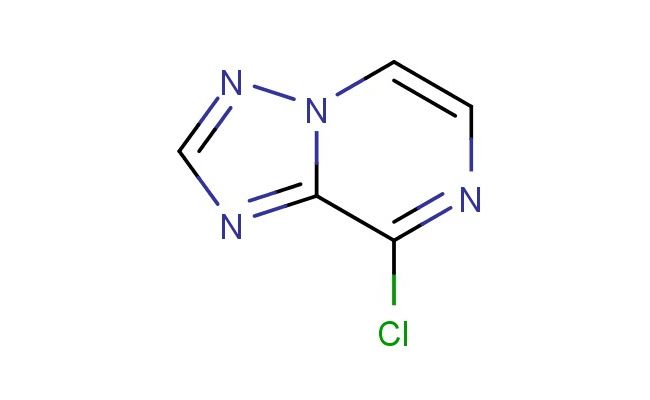
8-chloro-[1,2,4]triazolo[1,5-a]pyrazine
$400.00
CAS No.: 74803-32-0
Catalog No.: 196124
Purity: 95%
MF: C5H3ClN4
MW: 154.56
Storage: 2-8 degree Celsius
SMILES: ClC=1C=2N(C=CN1)N=CN2
Catalog No.: 196124
Purity: 95%
MF: C5H3ClN4
MW: 154.56
Storage: 2-8 degree Celsius
SMILES: ClC=1C=2N(C=CN1)N=CN2
For R&D use only. Not for human or animal use.
8-chloro-[1,2,4]triazolo[1,5-a]pyrazine; CAS No.: 74803-32-0; 8-chloro-[1,2,4]triazolo[1,5-a]pyrazine. PROPERTIES: This white to off-white crystalline powder has a molecular formula of C6H3ClN4 and a molecular weight of approximately 162.57 g/mol. It demonstrates moderate solubility in DMSO and DMF but is sparingly soluble in water. The compound is hygroscopic and should be stored in a desiccator at room temperature. Thermal decomposition occurs above 200 C. Safety guidelines recommend using P100 respirators, nitrile gloves, and splash goggles. In case of skin contact, wash with soap and water and remove contaminated clothing. Avoid inhaling dust as it may cause respiratory irritation. Do not release to the environment as chlorinated heterocycles may affect aquatic organisms. APPLICATIONS: 8-chloro-[1,2,4]triazolo[1,5-a]pyrazine serves as a valuable intermediate in pharmaceutical synthesis, particularly in the development of kinase inhibitors and immunomodulatory agents. Its chloro substituent enables nucleophilic aromatic substitution reactions for introducing diverse substituents. The triazolopyrazine core has demonstrated utility in developing JAK kinase inhibitors for autoimmune disease treatment. Research groups employ it in structure-activity relationship (SAR) studies to optimize binding affinity and selectivity. Academic institutions utilize it in teaching synthetic methodologies involving heterocyclic chemistry and electrophilic aromatic substitution. Industrial applications include its use as a building block in agrochemical development for novel herbicide candidates. Recent publications in Organic Letters highlight its role in transition metal-catalyzed coupling reactions for rapid library generation. Additionally, it finds utility in chemical biology as a warhead for covalent inhibitors targeting protein kinases. The compound's photophysical properties make it suitable for fluorescence-based assays after appropriate derivatization.
Reviews
Write Your Own Review



![6-bromo-[1,2,4]triazolo[1,5-a]pyrazine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196123_2.jpg)
![3-bromo-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/6/163978_1.jpg)