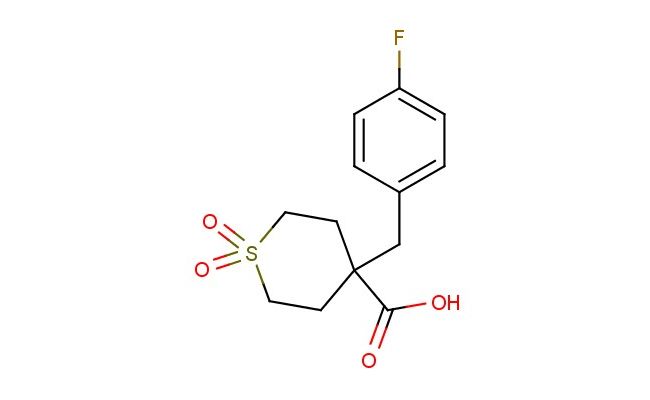
4-(4-fluorobenzyl)tetrahydro-2H-thiopyran-4-carboxylic acid 1,1-dioxide
$400.00
CAS No.: 1922897-51-5
Catalog No.: LT0029
Purity: 95%
MF: C13H15FO4S
MW: 286.324
Storage: 2-8 degree Celsius
SMILES: FC1=CC=C(CC2(CCS(CC2)(=O)=O)C(=O)O)C=C1
Catalog No.: LT0029
Purity: 95%
MF: C13H15FO4S
MW: 286.324
Storage: 2-8 degree Celsius
SMILES: FC1=CC=C(CC2(CCS(CC2)(=O)=O)C(=O)O)C=C1
For R&D use only. Not for human or animal use.
CAS NO.: 1922897-51-5;4-(4-fluorobenzyl)tetrahydro-2H-thiopyran-4-carboxylic acid 1,1-dioxide. PROPERTIES: This fluorinated sulfur-containing acid presents as a white crystalline solid with a molecular weight of approximately 269.3 g/mol. The 4-(4-fluorobenzyl)tetrahydro-2H-thiopyran-4-carboxylic acid 1,1-dioxide combines a fluorobenzyl group with a thiopyran sulfone framework. It exhibits limited aqueous solubility but good dissolution in polar aprotic solvents like DMSO and DMF. Stability characterization reveals sensitivity to base-catalyzed hydrolysis of the ester group and light exposure, necessitating storage at 2-8 degree Celsius in amber glass containers. Handlers should use powder hoods with HEPA filtration and wear cut-resistant gloves during handling. Skin contact may cause mild irritation requiring thorough washing. Inhalation may induce respiratory tract irritation; treatment includes fresh air and medical evaluation. Eye exposure requires extended rinsing and possible corticosteroid application. Waste should be hydrolyzed with dilute acid prior to disposal. APPLICATIONS: The 4-(4-fluorobenzyl)tetrahydro-2H-thiopyran-4-carboxylic acid 1,1-dioxide serves as a key intermediate in the synthesis of various pharmaceuticals. Its thiopyran sulfone framework provides opportunities for oxidative ring-opening reactions. Research teams utilize this compound as a starting material for creating kinase inhibitors and muscarinic receptor modulators. The fluorobenzyl group enhances metabolic resistance in resulting drug candidates. Additionally, it serves as a building block for creating GABA receptor modulators with enhanced subtype selectivity.
Reviews
Write Your Own Review