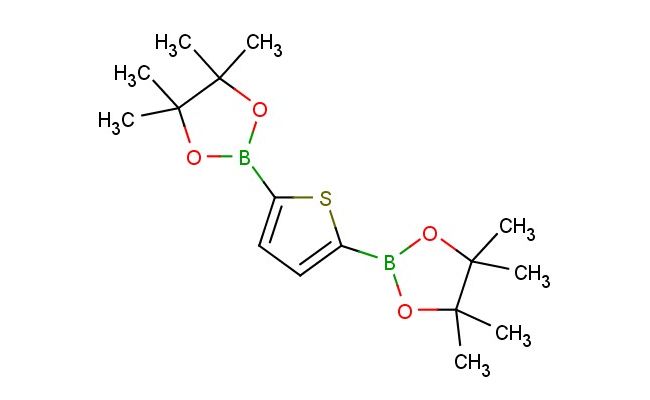
2,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophene
$200.00
CAS No.: 175361-81-6
Catalog No.: 196097
Purity: 95%
MF: C16H26B2O4S
MW: 336.071
Storage: 2-8 degree Celsius
SMILES: CC1(OB(OC1(C)C)C=1SC(=CC1)B1OC(C(O1)(C)C)(C)C)C
Catalog No.: 196097
Purity: 95%
MF: C16H26B2O4S
MW: 336.071
Storage: 2-8 degree Celsius
SMILES: CC1(OB(OC1(C)C)C=1SC(=CC1)B1OC(C(O1)(C)C)(C)C)C
For R&D use only. Not for human or animal use.
2,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophene; CAS No.: 175361-81-6; 2,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophene. PROPERTIES: 2,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophene is a crystalline powder with a molecular weight of approximately 482.4 g/mol. It has a melting point between 120-125 C. The compound is moderately soluble in common organic solvents like dimethylformamide and tetrahydrofuran but is practically insoluble in water. The dioxaborolane groups provide boron-containing linkages that are stable under neutral conditions. For proper storage, keep in a tightly sealed container at room temperature away from heat and moisture. Safety: This compound may cause serious eye irritation. In case of contact with eyes, rinse cautiously with water for several minutes and remove contact lenses if present. Use explosion-proof ventilation and lighting if handling large quantities. APPLICATIONS: In materials science, 2,5-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophene serves as a monomer for creating conjugated polymers via Suzuki-Miyaura coupling reactions. The dioxaborolane groups react with halogenated aromatic compounds to form extended -conjugated systems. In organic synthesis, it is used as a building block for creating bioactive molecules. The thiophene ring provides structural features that enhance binding to biological targets. In electronic materials, derivatives of this compound are explored for creating organic light-emitting diodes. The combination of the thiophene ring and the dioxaborolane groups creates materials with balanced hole and electron transport properties. These applications are supported by research in polymer chemistry journals and electronic materials publications.
Reviews
Write Your Own Review