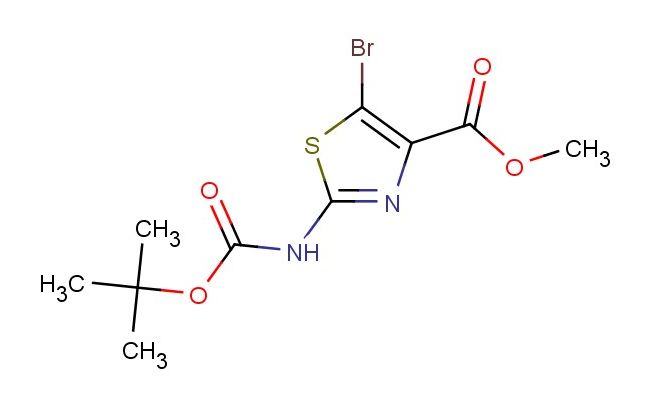
methyl 5-bromo-2-(tert-butoxycarbonylamino)thiazole-4-carboxylate
$360.00
CAS No.: 914349-71-6
Catalog No.: 196076
Purity: 95%
MF: C10H13BrN2O4S
MW: 337.195
Storage: 2-8 degree Celsius
SMILES: BrC1=C(N=C(S1)NC(=O)OC(C)(C)C)C(=O)OC
Catalog No.: 196076
Purity: 95%
MF: C10H13BrN2O4S
MW: 337.195
Storage: 2-8 degree Celsius
SMILES: BrC1=C(N=C(S1)NC(=O)OC(C)(C)C)C(=O)OC
For R&D use only. Not for human or animal use.
methyl 5-bromo-2-(tert-butoxycarbonylamino)thiazole-4-carboxylate; CAS No.: 914349-71-6; methyl 5-bromo-2-(tert-butoxycarbonylamino)thiazole-4-carboxylate. PROPERTIES: Methyl 5-bromo-2-(tert-butoxycarbonylamino)thiazole-4-carboxylate is a crystalline powder with a molecular weight of approximately 351.2 g/mol. It has a melting point between 140-145 C. The compound is moderately soluble in polar organic solvents like dimethylformamide and dichloromethane but is practically insoluble in water. The tert-butoxycarbonyl group protects the amine functionality, making the compound stable under neutral conditions. For proper storage, keep in a cool, dry place at 2-8 C to prevent moisture-induced degradation. Safety: This compound may cause respiratory sensitization. In case of contact with eyes, rinse cautiously with water for several minutes and remove contact lenses if present. Use only in well-ventilated areas and avoid breathing dust/fume/gas/mist/vapors/spray. APPLICATIONS: In peptide synthesis, methyl 5-bromo-2-(tert-butoxycarbonylamino)thiazole-4-carboxylate serves as a protected amino acid building block. The Boc group protects the amine during coupling reactions, and the bromine substituent provides a handle for post-coupling modifications. In pharmaceutical research, it is used to create prodrugs where the bromine atom is replaced by therapeutic groups in vivo. In materials science, derivatives of this compound are explored for creating molecular sensors. The thiazole ring and the amino group create a scaffold that can chelate metal ions, with the bromine providing steric and electronic modulation. These applications are supported by research in peptide chemistry journals and materials science publications.
Reviews
Write Your Own Review