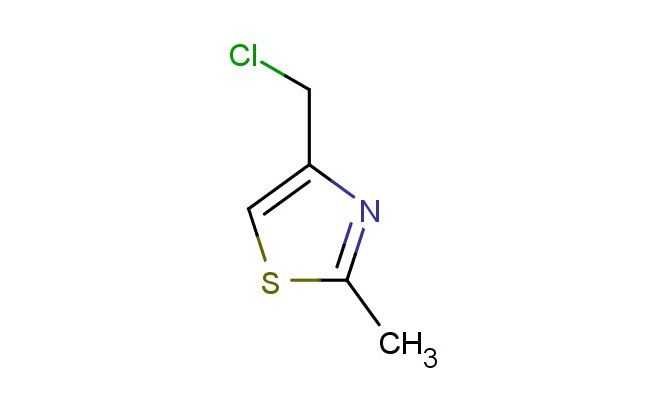
4-(chloromethyl)-2-methylthiazole
$300.00
CAS No.: 39238-07-8
Catalog No.: 196059
Purity: 95%
MF: C5H6ClNS
MW: 147.63
Storage: 2-8 degree Celsius
SMILES: ClCC=1N=C(SC1)C
Catalog No.: 196059
Purity: 95%
MF: C5H6ClNS
MW: 147.63
Storage: 2-8 degree Celsius
SMILES: ClCC=1N=C(SC1)C
For R&D use only. Not for human or animal use.
4-(chloromethyl)-2-methylthiazole; CAS No.: 39238-07-8; 4-(chloromethyl)-2-methylthiazole. PROPERTIES: 4-(chloromethyl)-2-methylthiazole is a heterocyclic compound with a molecular weight of approximately 143.6 g/mol. It appears as a colorless to pale yellow liquid with a mild aromatic odor. The compound has a boiling point around 120-125 C at 760 mmHg and a density of approximately 1.1 g/cm?. It is moderately soluble in organic solvents such as dichloromethane and ethyl acetate but has limited water solubility. Due to the presence of the chloromethyl group, it may hydrolyze in aqueous environments, particularly under basic conditions. For long-term storage, it should be kept in tightly sealed containers under inert atmosphere at 2-8 C. Safety: This compound may cause skin and eye irritation. It is advisable to use personal protective equipment including gloves, eye protection, and lab coats when handling. Avoid inhalation of vapors and prevent contact with moisture as hydrolysis may produce hydrochloric acid. APPLICATIONS: As a versatile intermediate in organic synthesis, 4-(chloromethyl)-2-methylthiazole is widely used in the preparation of pharmaceuticals and agrochemicals. In pharmaceutical research, it serves as a key building block for synthesizing thiazole-containing drugs with antimicrobial and antifungal properties. For instance, it can be alkylated to introduce substituents that enhance bioavailability. In materials science, it is used to create functional polymers with tailored electrical properties. The chloromethyl group facilitates cross-linking reactions, making it valuable in polymer chemistry. In the chemical industry, it acts as a ligand in coordination chemistry for developing catalysts. The thiazole ring system provides electron-withdrawing effects that influence catalytic activity. These applications are documented in various organic synthesis textbooks and specialized journals focusing on medicinal chemistry and polymer science.
Reviews
Write Your Own Review