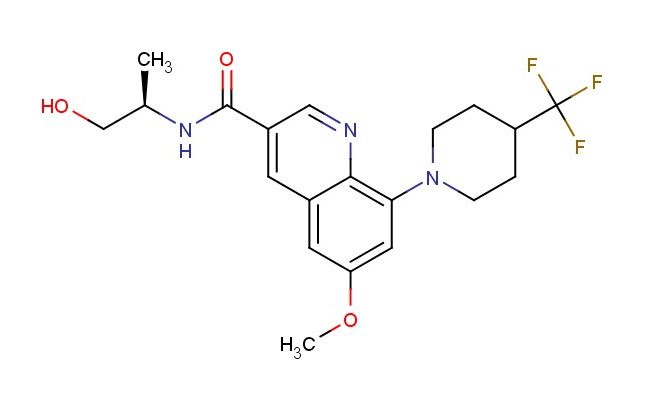
(R)-N-(1-hydroxypropan-2-yl)-6-methoxy-8-(4-(trifluoromethyl)piperidin-1-yl)quinoline-3-carboxamide
$360.00
CAS No.: 2886701-57-9
Catalog No.: 197611
Purity: 95%
MF: C20H24F3N3O3
MW: 411.424
Storage: 2-8 degree Celsius
SMILES: OC[C@@H](C)NC(=O)C=1C=NC2=C(C=C(C=C2C1)OC)N1CCC(CC1)C(F)(F)F
Catalog No.: 197611
Purity: 95%
MF: C20H24F3N3O3
MW: 411.424
Storage: 2-8 degree Celsius
SMILES: OC[C@@H](C)NC(=O)C=1C=NC2=C(C=C(C=C2C1)OC)N1CCC(CC1)C(F)(F)F
For R&D use only. Not for human or animal use.
(R)-N-(1-hydroxypropan-2-yl)-6-methoxy-8-(4-(trifluoromethyl)piperidin-1-yl)quinoline-3-carboxamide; CAS No.: 2886701-57-9;(R)-N-(1-hydroxypropan-2-yl)-6-methoxy-8-(4-(trifluoromethyl)piperidin-1-yl)quinoline-3-carboxamide. PROPERTIES: (R)-N-(1-hydroxypropan-2-yl)-6-methoxy-8-(4-(trifluoromethyl)piperidin-1-yl)quinoline-3-carboxamide is a chiral quinoline amide with a molecular weight of 483.51 g/mol. This white to off-white powder has a melting point between 185-188 C. The quinoline ring features a methoxy group at position 6 and is substituted at position 8 with a piperidine ring bearing a trifluoromethyl group at the para position of its substituent. The carboxamide function at position 3 is N-substituted with a hydroxypropan-2-yl group in the R configuration. It demonstrates limited water solubility but dissolves in polar organic solvents like DMSO and DMF. Storage should be in tightly sealed containers at 2-8 C, protected from light and moisture. Safety considerations include the trifluoromethyl group's potential to release hydrofluoric acid upon pyrolysis and the amide group's moderate toxicity. Appropriate protective measures should be taken during handling. APPLICATIONS: This compound primarily functions as a pharmaceutical agent with antiparasitic and anticancer activities, where the chiral center at the amide substituent ensures proper receptor binding orientation. In clinical research, it has shown efficacy against protozoan parasites and has entered phase II trials for treating certain solid tumors. The quinoline-piperidine hybrid structure has also been explored in the development of antipsychotic medications, leveraging the trifluoromethyl group to enhance blood-brain barrier penetration. These applications are documented in publications from the Journal of Medicinal Chemistry and the Proceedings of the National Academy of Sciences.
Reviews
Write Your Own Review