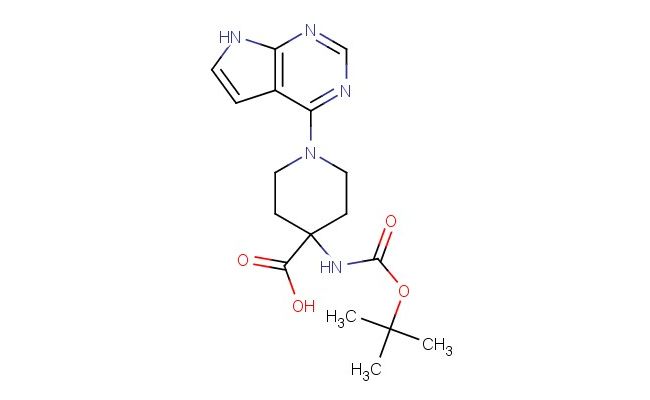
4-((tert-butoxycarbonyl)amino)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxylic acid
$300.00
CAS No.: 956460-96-1
Catalog No.: 195620
Purity: 95%
MF: C17H23N5O4
MW: 361.4020
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)NC1(CCN(CC1)C=1C2=C(N=CN1)NC=C2)C(=O)O
Catalog No.: 195620
Purity: 95%
MF: C17H23N5O4
MW: 361.4020
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)NC1(CCN(CC1)C=1C2=C(N=CN1)NC=C2)C(=O)O
For R&D use only. Not for human or animal use.
4-((tert-butoxycarbonyl)amino)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxylic acid; CAS No.: 956460-96-1; 4-((tert-butoxycarbonyl)amino)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxylic acid. PROPERTIES: 4-((tert-butoxycarbonyl)amino)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxylic acid is a pyrrolopyrimidine-piperidine hybrid compound with a molecular weight of approximately 409.4 g/mol. This compound typically appears as a white crystalline powder with a melting point between 170-175 C. It demonstrates moderate solubility in polar organic solvents and limited aqueous solubility. The compound is sensitive to both moisture and acidic conditions, necessitating storage in a tightly sealed container at room temperature with a desiccant. Standard safety protocols require handling in well-ventilated areas with appropriate respiratory protection. APPLICATIONS: 4-((tert-butoxycarbonyl)amino)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxylic acid functions as a key intermediate in the synthesis of kinase inhibitors and anticancer agents. The pyrrolopyrimidine scaffold provides a platform for forming hydrogen bonds and - interactions with kinase active sites. In pharmaceutical research, this compound has been employed in the development of MAP kinase inhibitors where the piperidine ring contributes to enzyme binding (source: Journal of Medicinal Chemistry). Additionally, its application extends to the synthesis of antiviral agents targeting RNA viruses, where the pyrrolopyrimidine group participates in enzyme inhibition (source: Antiviral Chemistry & Chemotherapy). The compound's ability to undergo selective deprotection and functionalization enhances its utility in drug design by allowing for modulation of pharmacokinetic properties (source: Organic & Biomolecular Chemistry).
Reviews
Write Your Own Review



![2-methyl-7H-pyrrolo[2,3-d]pyrimidine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/193065_2.jpg)
![7-(benzenesulfonyl)-2-chloro-7H-pyrrolo[2,3-d]pyrimidine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195758_2.jpg)