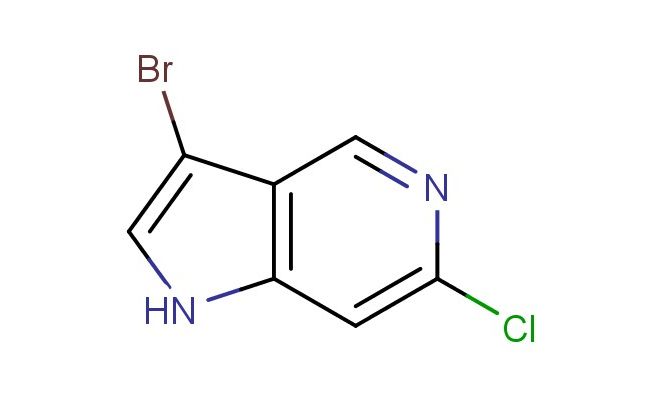
3-bromo-6-chloro-1H-pyrrolo[3,2-c]pyridine
$360.00
CAS No.: 1000341-61-6
Catalog No.: 195757
Purity: 95%
MF: C7H4BrClN2
MW: 231.48
Storage: 2-8 degree Celsius
SMILES: BrC1=CNC2=C1C=NC(=C2)Cl
Catalog No.: 195757
Purity: 95%
MF: C7H4BrClN2
MW: 231.48
Storage: 2-8 degree Celsius
SMILES: BrC1=CNC2=C1C=NC(=C2)Cl
For R&D use only. Not for human or animal use.
3-bromo-6-chloro-1H-pyrrolo[3,2-c]pyridine; CAS No.: 1000341-61-6; 3-bromo-6-chloro-1H-pyrrolo[3,2-c]pyridine. PROPERTIES: 3-Bromo-6-chloro-1H-pyrrolo[3,2-c]pyridine has molecular formula C8H4BrClN2, giving it a molecular weight of 262.48 g/mol. It appears as a white crystalline solid with a melting point between 165-168 C. The compound demonstrates good chemical stability under standard conditions but is sensitive to strong nucleophilic attack. Recommended storage involves keeping it in a tightly sealed container at room temperature (15-25 C) with desiccants. Safety data indicates it may cause skin irritation and has a flash point of approximately 85 C. The compound has a logP value of approximately 2.0 and exhibits limited aqueous solubility. APPLICATIONS: This 3-bromo-6-chloro-1H-pyrrolo[3,2-c]pyridine is extensively used in modern pharmaceutical synthesis as a cross-coupling partner. The bromo and chloro substituents enable efficient Suzuki-Miyaura and Buchwald-Hartwig amination reactions to form complex polyaryl architectures common in kinase inhibitors. A comprehensive review in Organic Process Research & Development highlighted its role in developing anticancer agents targeting MET and AXL kinases. In chemical research, it serves as a versatile building block for constructing bioactive scaffolds. The bromo and chloro groups provide orthogonal reactivity for sequential coupling reactions. Additionally, the compound is utilized in the preparation of pyrrolopyridine-containing radiotracers. Research in Bioconjugate Chemistry demonstrated its utility in creating iodine-123 labeled imaging agents for visualizing neuroreceptor densities in psychiatric disorders. The chloro substituent offers a site for further functionalization to modulate tissue penetration properties.
Reviews
Write Your Own Review



![3-bromo-1H-pyrrolo[2,3-b]pyridine-4-carboxylic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195756_2.jpg)
![1H-pyrrolo[2,3-b]pyridin-3-amine hydrochloride](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196487_2.jpg)