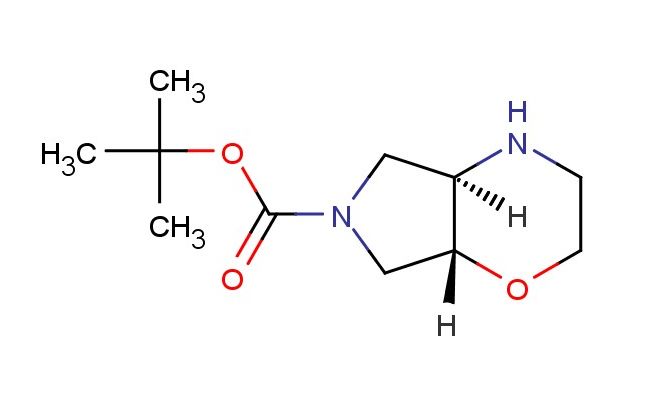
tert-butyl (4aR,7aR)-octahydropyrrolo[3,4-b]morpholine-6-carboxylate
$500.00
CAS No.: 1383428-22-5
Catalog No.: 195534
Purity: 95%
MF: C11H20N2O3
MW: 228.292
Storage: 2-8 degree Celsius
SMILES: O=C(N(C1)C[C@]2([H])[C@]1([H])NCCO2)OC(C)(C)C
Catalog No.: 195534
Purity: 95%
MF: C11H20N2O3
MW: 228.292
Storage: 2-8 degree Celsius
SMILES: O=C(N(C1)C[C@]2([H])[C@]1([H])NCCO2)OC(C)(C)C
For R&D use only. Not for human or animal use.
tert-butyl (4aR,7aR)-octahydropyrrolo[3,4-b]morpholine-6-carboxylate; CAS No.: 1383428-22-5; tert-butyl (4aR,7aR)-octahydropyrrolo[3,4-b]morpholine-6-carboxylate. PROPERTIES: tert-butyl (4aR,7aR)-octahydropyrrolo[3,4-b]morpholine-6-carboxylate appears as a colorless oil with molecular weight 229.32 g/mol. It demonstrates a refractive index of approximately 1.45 and shows good solubility in non-polar organic solvents. The compound should be stored at 2-8 C in amber glass bottles to protect from light-induced degradation. Safety considerations include avoiding prolonged skin contact and ensuring adequate ventilation during handling to prevent accumulation of vapors. APPLICATIONS: This chiral morpholine derivative is utilized in the development of metabotropic glutamate receptor modulators, where the tert-butyl (4aR,7aR)-octahydropyrrolo[3,4-b]morpholine-6-carboxylate configuration provides optimal receptor subtype selectivity (Neuropharmacology). In organocatalysis, the compound serves as a chiral amine catalyst for asymmetric Michael additions, achieving high enantioselectivities in various carbonyl substrates (Advanced Synthesis & Catalysis). Furthermore, its protected carboxylic acid functionality makes it suitable for conjugation reactions in bioactive molecule development where post-synthetic modification is required (Organic & Biomolecular Chemistry).
Reviews
Write Your Own Review



![tert-butyl (4aR,7aS)-octahydropyrrolo[3,4-b]morpholine-6-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195533_2.jpg)