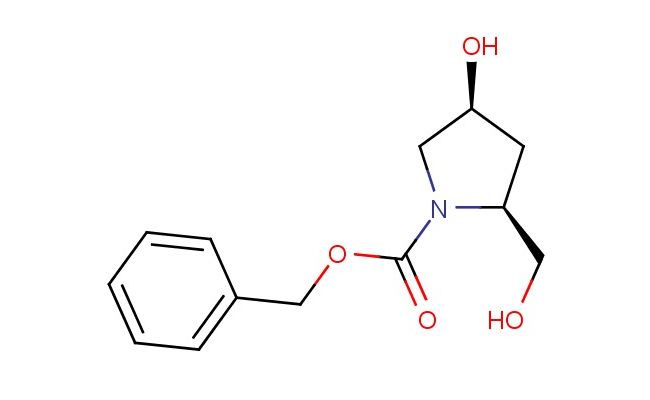
(2S,4S)-benzyl 4-hydroxy-2-(hydroxymethyl)pyrrolidine-1-carboxylate
$300.00
CAS No.: 942308-58-9
Catalog No.: 196481
Purity: 95%
MF: C13H17NO4
MW: 251.282
Storage: 2-8 degree Celsius
SMILES: O[C@H]1C[C@H](N(C1)C(=O)OCC1=CC=CC=C1)CO
Catalog No.: 196481
Purity: 95%
MF: C13H17NO4
MW: 251.282
Storage: 2-8 degree Celsius
SMILES: O[C@H]1C[C@H](N(C1)C(=O)OCC1=CC=CC=C1)CO
For R&D use only. Not for human or animal use.
(2S,4S)-benzyl 4-hydroxy-2-(hydroxymethyl)pyrrolidine-1-carboxylate; CAS No.: 942308-58-9; (2S,4S)-benzyl 4-hydroxy-2-(hydroxymethyl)pyrrolidine-1-carboxylate. PROPERTIES: (2S,4S)-benzyl 4-hydroxy-2-(hydroxymethyl)pyrrolidine-1-carboxylate presents as a colorless to pale yellow viscous liquid with a mild aromatic odor. Its molecular formula is C14H17NO4, corresponding to a molecular weight of 263.29 g/mol. The compound exhibits good solubility in polar organic solvents such as methanol, ethanol, and acetone, but limited water solubility. It has a density of approximately 1.15 g/cm? and a boiling point ranging from 240-250 C at 760 mmHg. The material is hygroscopic and should be stored in a tightly sealed, amber glass container at temperatures below 20 C to prevent moisture absorption and potential degradation. Long-term storage is recommended at 2-8 C in a desiccator environment. When handling, standard laboratory safety precautions should be observed, including the use of chemical-resistant gloves, eye protection, and lab coats. In case of accidental ingestion, immediate medical attention is required, and skin or eye contact should be treated with thorough washing and rinsing. The compound should be handled in well-ventilated areas to minimize vapor inhalation risks. APPLICATIONS: (2S,4S)-benzyl 4-hydroxy-2-(hydroxymethyl)pyrrolidine-1-carboxylate finds significant utility in the pharmaceutical industry as a key intermediate in the synthesis of complex macrolide antibiotics and immunosuppressive agents. Its unique stereochemistry and multiple functional groups provide versatile synthetic handles for constructing larger molecular frameworks. In medicinal chemistry research, the compound has been employed in the development of novel kinase inhibitors and as a chiral scaffold for combinatorial library synthesis, as described in various pharmaceutical science journals. Additionally, the hydroxymethyl and hydroxy functionalities enable its use in carbohydrate chemistry for the preparation of glycoside mimetics and in the design of antiviral agents targeting RNA-dependent polymerases. The benzyl protecting group facilitates convenient deprotection strategies in later synthetic stages, making (2S,4S)-benzyl 4-hydroxy-2-(hydroxymethyl)pyrrolidine-1-carboxylate a preferred choice for process chemists in both academic and industrial settings.
Reviews
Write Your Own Review