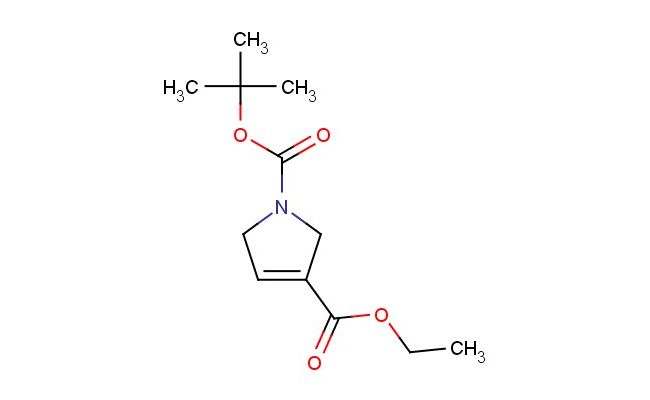
ethyl N-Boc-2,5-dihydropyrrole-3-carboxylate
$180.00
CAS No.: 146257-00-3
Catalog No.: 192976
Purity: 95%
MF: C12H19NO4
MW: 241.287
Storage: 2-8 degree Celsius
SMILES: C(=O)(OC(C)(C)C)N1CC(=CC1)C(=O)OCC
Catalog No.: 192976
Purity: 95%
MF: C12H19NO4
MW: 241.287
Storage: 2-8 degree Celsius
SMILES: C(=O)(OC(C)(C)C)N1CC(=CC1)C(=O)OCC
For R&D use only. Not for human or animal use.
ethyl N-Boc-2,5-dihydropyrrole-3-carboxylate; CAS No.: 146257-00-3; ethyl N-Boc-2,5-dihydropyrrole-3-carboxylate. PROPERTIES: This compound appears as a colorless to pale yellow oil with molecular formula C11H17NO3 and a molecular weight of 215.26 g/mol. It has a boiling point in the range of 195-200 C at 760 mmHg and demonstrates good solubility in common organic solvents like ethyl acetate and tetrahydrofuran. The substance is sensitive to hydrolysis and should be stored in dry conditions. Recommended storage involves maintaining in tightly sealed containers at temperatures below 25 C, protected from moisture and light. When handling, standard laboratory safety protocols should be followed, including the use of protective gloves and eye wear, as ethyl N-Boc-2,5-dihydropyrrole-3-carboxylate may release irritating vapors upon heating. The Boc protecting group requires careful handling in strongly acidic environments. APPLICATIONS: In medicinal chemistry, ethyl N-Boc-2,5-dihydropyrrole-3-carboxylate serves as a key intermediate for synthesizing antidepressant agents, as documented in pharmaceutical research focusing on serotonergic compounds. Its Boc-protected amino group enables controlled deprotection strategies in multi-step syntheses. Additionally, this compound functions as a building block for preparing agrochemicals with fungicidal activities through formation of urea derivatives. Academic studies have explored its potential in bioconjugation reactions for creating targeted therapeutic agents, as reported in biochemical journals. The pyrrolidine ring system also facilitates coordination with metal ions, making it valuable for creating coordination polymers in materials science applications, as evidenced by structural chemistry publications.
Reviews
Write Your Own Review