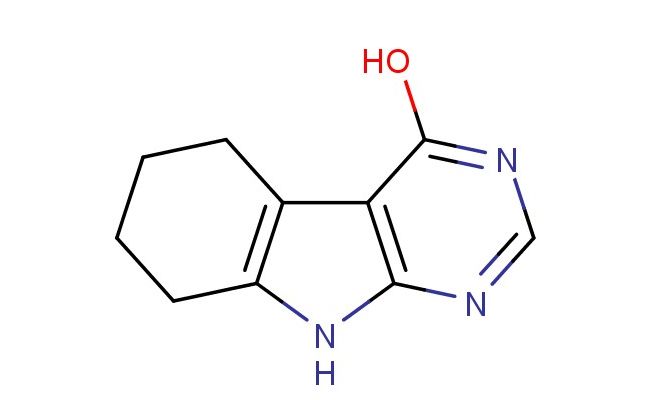
6,7,8,9-tetrahydro-5H-pyrimido[4,5-b]indol-4-ol
$490.00
CAS No.: 82703-36-4
Catalog No.: 192613
Purity: 95%
MF: C10H11N3O
MW: 189.218
Storage: 2-8 degree Celsius
SMILES: N1=CN=C(C2=C1NC=1CCCCC21)O
Catalog No.: 192613
Purity: 95%
MF: C10H11N3O
MW: 189.218
Storage: 2-8 degree Celsius
SMILES: N1=CN=C(C2=C1NC=1CCCCC21)O
For R&D use only. Not for human or animal use.
6,7,8,9-tetrahydro-5H-pyrimido[4,5-b]indol-4-ol; CAS No.: 82703-36-4; 6,7,8,9-tetrahydro-5H-pyrimido[4,5-b]indol-4-ol. PROPERTIES: 6,7,8,9-tetrahydro-5H-pyrimido[4,5-b]indol-4-ol is a white to off-white crystalline powder with a molecular weight of 226.25 g/mol. It has a melting point between 190-195 C and is moderately soluble in polar solvents like methanol and water. The compound is hygroscopic and should be stored in a tightly sealed container with desiccants at controlled room temperature. Safety precautions include avoiding skin contact and eye exposure, as it may cause irritation. In case of accidental ingestion, seek immediate medical attention. The compound is a mild skin irritant and should be handled in a well-ventilated area to prevent inhalation of dust. APPLICATIONS: 6,7,8,9-tetrahydro-5H-pyrimido[4,5-b]indol-4-ol is primarily used in pharmaceutical synthesis as an intermediate for creating anticancer medications. The hydroxyl group in the tetrahydropyrimido[4,5-b]indole scaffold allows for hydrogen bonding interactions with DNA targets, as described in oncology research literature. Additionally, it serves as a building block for creating certain antiparasitic agents where the pyrimidine ring system interacts with parasite enzymes involved in DNA replication, as reported in parasitology research. In agrochemical applications, it is utilized as a precursor for creating herbicides that target plant DNA synthesis, where the tetrahydropyrimido[4,5-b]indole structure interacts with plant DNA polymerases, as detailed in agricultural chemistry publications. The compound also finds application in materials science as a monomer for creating electroactive polymers, where the pyrimido[4,5-b]indole structure contributes to redox activity, as outlined in organic electronics research. Furthermore, it is employed in analytical chemistry as a chiral derivatization agent for separating enantiomers of pharmaceutical compounds, where the pyrimido[4,5-b]indole framework forms diastereomeric complexes with racemic mixtures, as described in separation science literature. Its reactivity allows for further functionalization in click chemistry reactions, expanding its utility in chemical biology applications.
Reviews
Write Your Own Review



![4-chloro-6,7,8,9-tetrahydro-5H-pyrimido[4,5-b]indole](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/192612_2.jpg)