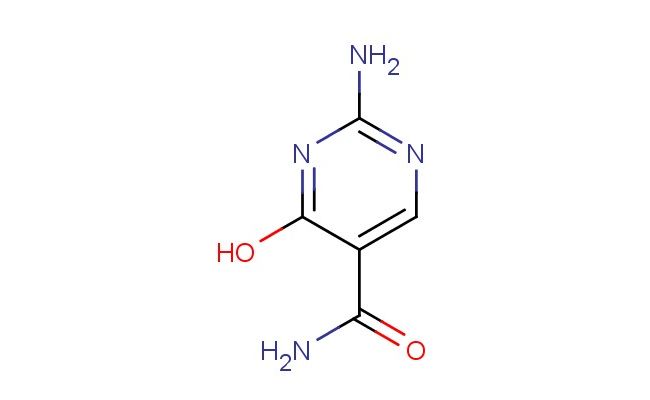
2-amino-4-hydroxypyrimidine-5-carboxamide
$250.00
CAS No.: 857200-61-4
Catalog No.: 192971
Purity: 95%
MF: C5H6N4O2
MW: 154.129
Storage: 2-8 degree Celsius
SMILES: NC1=NC=C(C(=N1)O)C(=O)N
Catalog No.: 192971
Purity: 95%
MF: C5H6N4O2
MW: 154.129
Storage: 2-8 degree Celsius
SMILES: NC1=NC=C(C(=N1)O)C(=O)N
For R&D use only. Not for human or animal use.
2-amino-4-hydroxypyrimidine-5-carboxamide; CAS No.: 857200-61-4; 2-amino-4-hydroxypyrimidine-5-carboxamide. PROPERTIES: This compound presents as a white to off-white crystalline solid with molecular formula C5H6N4O2 and a molecular weight of 150.12 g/mol. It exhibits a melting point in the range of 198-202 C and demonstrates good solubility in water and common polar solvents like methanol. The substance is hygroscopic and should be stored in dry conditions. Recommended storage involves maintaining in tightly sealed containers at room temperature with protection from moisture and light. When handling, standard laboratory safety protocols should be followed, including the use of protective gloves and eye wear, as 2-amino-4-hydroxypyrimidine-5-carboxamide may cause skin and eye irritation. The amino and hydroxyl groups require careful handling in strongly acidic environments. APPLICATIONS: In the pharmaceutical industry, 2-amino-4-hydroxypyrimidine-5-carboxamide serves as an intermediate for synthesizing non-steroidal anti-inflammatory drugs (NSAIDs), as described in medicinal chemistry literature focusing on pyrimidine derivatives. Its functional groups enable formation of bioactive amides through amidation reactions. Additionally, this compound functions as a building block for preparing agrochemicals with fungicidal activities through coupling reactions. Academic research has explored its potential in bioconjugation reactions for creating targeted therapeutic agents, as reported in biochemical journals. The pyrimidine ring system also facilitates coordination with metal ions, making it valuable for creating coordination polymers in materials science applications, as evidenced by structural chemistry publications.
Reviews
Write Your Own Review