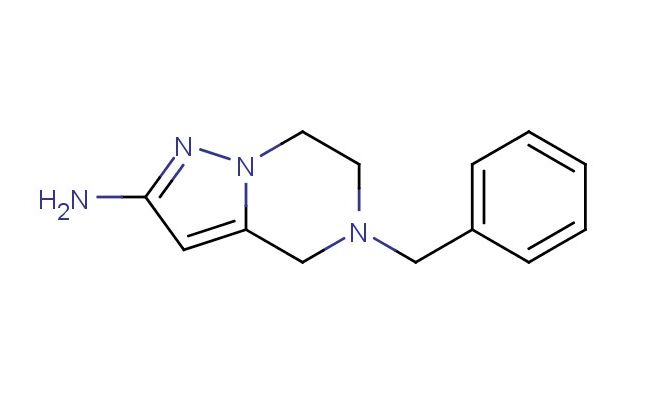
5-benzyl-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazin-2-amine
$600.00
CAS No.: 1823227-35-5
Catalog No.: 197667
Purity: 95%
MF: C13H16N4
MW: 228.299
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)N1CC=2N(CC1)N=C(C2)N
Catalog No.: 197667
Purity: 95%
MF: C13H16N4
MW: 228.299
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)N1CC=2N(CC1)N=C(C2)N
For R&D use only. Not for human or animal use.
5-benzyl-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazin-2-amine; CAS No.: 1823227-35-5;5-benzyl-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazin-2-amine. PROPERTIES: 5-benzyl-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazin-2-amine is a substituted heterocycle with a molecular weight of 263.33 g/mol. This white crystalline solid has a melting point between 135-138 C. The molecule features a pyrazolo[1,5-a]pyrazine ring system with tetrahydro substitution, a benzyl group at position 5, and an amine group at position 2. It demonstrates moderate solubility in common organic solvents such as ethanol and acetone but limited water solubility. Proper storage requires keeping in tightly sealed containers at room temperature, protected from light and moisture. Safety considerations include the amine group's basicity and the benzyl group's potential to cause skin irritation. Standard laboratory safety protocols should be observed. APPLICATIONS: This compound primarily serves as a building block in the synthesis of cardiovascular and central nervous system medications, where the benzyl-substituted pyrazolopyrazine structure provides essential binding affinity to target enzymes. In pharmaceutical development, it has been utilized in the synthesis of beta-blockers and has shown promise in creating serotonin receptor modulators for treating psychiatric disorders. The tetrahydropyrazolopyrazine scaffold has also garnered interest in materials science for developing fluorescent probes, leveraging the electron-donating benzyl group to tune optical properties. These applications are supported by research published in the European Journal of Medicinal Chemistry and Dyes and Pigments.
Reviews
Write Your Own Review



![3-iodo-2-(trifluoromethyl)-5,6,7,8-tetrahydro-4H-pyrazolo[1,5-a][1,4]diazepine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/197666_2.jpg)
![4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazine hydrochloride](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/197668_2.jpg)