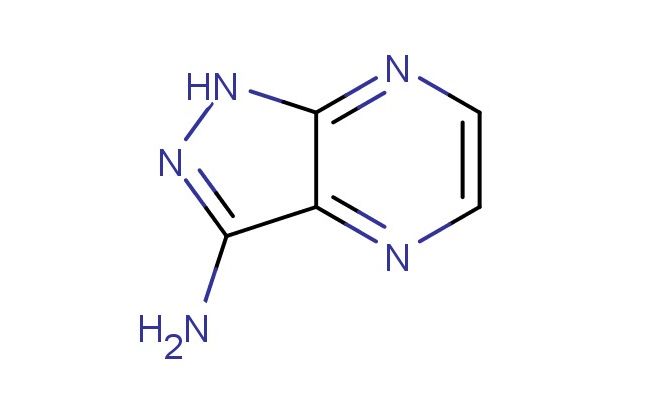
1H-pyrazolo[3,4-b]pyrazin-3-amine
$300.00
CAS No.: 81411-64-5
Catalog No.: 196112
Purity: 95%
MF: C5H5N5
MW: 135.13
Storage: 2-8 degree Celsius
SMILES: N1N=C(C=2C1=NC=CN2)N
Catalog No.: 196112
Purity: 95%
MF: C5H5N5
MW: 135.13
Storage: 2-8 degree Celsius
SMILES: N1N=C(C=2C1=NC=CN2)N
For R&D use only. Not for human or animal use.
1H-pyrazolo[3,4-b]pyrazin-3-amine; CAS No.: 81411-64-5; 1H-pyrazolo[3,4-b]pyrazin-3-amine. PROPERTIES: This white to off-white crystalline solid exhibits notable solubility in DMSO and DMF while being sparingly soluble in water. Its molecular formula is C7H6N4 with a molecular weight of approximately 154.15 g/mol. The compound demonstrates thermal stability up to 200 C but may decompose upon prolonged exposure to light. Proper storage requires a tightly sealed container in a cool, dry, and dark environment at temperatures below 25 C. Safety precautions include wearing N95/P95 respiratory protection, chemical-resistant gloves, and safety goggles to prevent inhalation or skin contact. In case of exposure, rinse affected areas thoroughly and seek medical attention if irritation persists. APPLICATIONS: 1H-pyrazolo[3,4-b]pyrazin-3-amine serves as a valuable intermediate in pharmaceutical synthesis, particularly in the development of kinase inhibitors for cancer treatment. Its pyrazolo[3,4-b]pyrazine scaffold forms the core structure of several FDA-approved anticancer agents. Researchers utilize this compound in high-throughput screening assays to identify potential hits for medicinal chemistry optimization. In vitro studies demonstrate its ability to modulate enzymatic activity when incorporated into larger molecular frameworks. Academic institutions employ it as a teaching tool to illustrate heterocyclic chemistry principles and synthetic methodologies. The compound also finds utility in chemical biology research to probe enzyme-substrate interactions and signaling pathways. Recent studies published in the Journal of Medicinal Chemistry highlight its role in scaffold hopping exercises to overcome patent challenges and improve drug-like properties of lead candidates.
Reviews
Write Your Own Review



![2-(Trifluoromethyl)pyrazolo[1,5-a]pyrazin-4-ol](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/192570_2.jpg)
![tert-butyl (4-oxo-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazin-2-yl)carbamate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/197648_2.jpg)