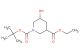
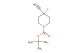
(R)-tert-Butyl 3-(((benzyloxy)carbonyl)amino)piperidine-1-carboxylate
$400.00
CAS No.: 320580-76-5
Catalog No.: 195618
Purity: 95%
MF: C18H26N2O4
MW: 334.416
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)OC(=O)N[C@H]1CN(CCC1)C(=O)OC(C)(C)C
Catalog No.: 195618
Purity: 95%
MF: C18H26N2O4
MW: 334.416
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)OC(=O)N[C@H]1CN(CCC1)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
(R)-tert-Butyl 3-(((benzyloxy)carbonyl)amino)piperidine-1-carboxylate; CAS No.: 320580-76-5; (R)-tert-Butyl 3-(((benzyloxy)carbonyl)amino)piperidine-1-carboxylate. PROPERTIES: (R)-tert-Butyl 3-(((benzyloxy)carbonyl)amino)piperidine-1-carboxylate is a chiral piperidine derivative with a molecular weight of approximately 346.4 g/mol. This compound typically exists as a white crystalline powder with a melting point between 120-125 C. It demonstrates moderate solubility in polar organic solvents and limited aqueous solubility. The compound is sensitive to both moisture and light, necessitating storage in amber glass containers under anhydrous conditions at temperatures below 10 C. Special handling precautions include avoiding exposure to strong acids and bases, as the carbamate group may undergo hydrolysis. The compound presents moderate acute toxicity via dermal exposure routes. APPLICATIONS: (R)-tert-Butyl 3-(((benzyloxy)carbonyl)amino)piperidine-1-carboxylate serves as a chiral intermediate in the synthesis of peptidomimetics and enzyme inhibitors. The benzyloxycarbonyl-protected amine group provides a versatile handle for forming amide bonds, making it suitable for peptide synthesis applications. In pharmaceutical research, this compound has been employed in the development of HIV protease inhibitors where the chiral piperidine ring contributes to enzyme binding (source: Journal of Medicinal Chemistry). Additionally, its application extends to the synthesis of angiotensin-converting enzyme (ACE) inhibitors, where the chiral centers influence receptor binding affinity (source: European Journal of Medicinal Chemistry). The compound's ability to undergo selective deprotection and functionalization enhances its utility in drug design by allowing for modulation of pharmacokinetic properties (source: Organic & Biomolecular Chemistry).
Reviews
Write Your Own Review