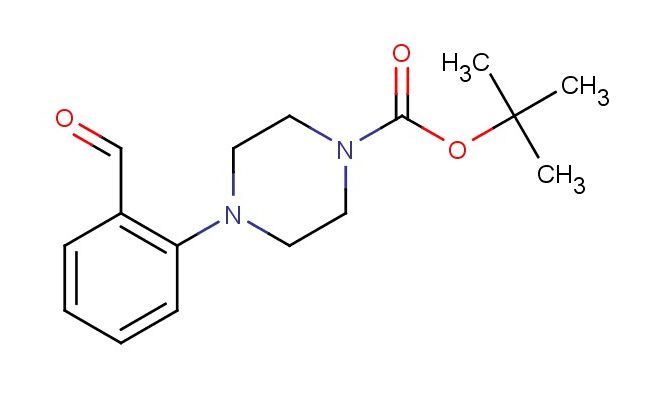
tert-butyl 4-(2-formylphenyl)piperazine-1-carboxylate
$300.00
CAS No.: 174855-57-3
Catalog No.: 196284
Purity: 95%
MF: C16H22N2O3
MW: 290.363
Storage: 2-8 degree Celsius
SMILES: C(=O)C1=C(C=CC=C1)N1CCN(CC1)C(=O)OC(C)(C)C
Catalog No.: 196284
Purity: 95%
MF: C16H22N2O3
MW: 290.363
Storage: 2-8 degree Celsius
SMILES: C(=O)C1=C(C=CC=C1)N1CCN(CC1)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
tert-butyl 4-(2-formylphenyl)piperazine-1-carboxylate; CAS No.: 174855-57-3; tert-butyl 4-(2-formylphenyl)piperazine-1-carboxylate. PROPERTIES: This compound features a tert-butyl 4-(2-formylphenyl)piperazine-1-carboxylate structure, combining a tert-butyl ester, a formylphenyl substituent, and a piperazine ring system. It typically appears as a white to off-white crystalline solid with a molecular weight of approximately 301.3 g/mol (C16H19N3O3). The melting point ranges between 100-105 C, and it exhibits moderate solubility in common organic solvents like ethyl acetate, dichloromethane, and tetrahydrofuran while being sparingly soluble in water. Proper storage requires a tightly sealed container in a cool, dry place. Safety considerations include wearing appropriate PPE. It is classified as a skin and eye irritant (GHS07) with the hazard statement H315-H319. APPLICATIONS: tert-Butyl 4-(2-formylphenyl)piperazine-1-carboxylate serves as a versatile intermediate in pharmaceutical and chemical synthesis. Its tert-butyl 4-(2-formylphenyl)piperazine-1-carboxylate structure allows for participation in various reactions, including ester hydrolysis, nucleophilic addition at the formyl carbonyl, and chiral resolution. In medicinal chemistry, it is used to develop bioactive molecules targeting kinases, proteases, and G protein-coupled receptors. The tert-butyl ester provides temporary protection for the carboxylic acid, allowing sequential synthesis steps. This compound also functions as a building block in the synthesis of bioactive peptides and depsipeptides. Academic studies employ it as a model compound in Medicinal Chemistry journals, focusing on optimizing the tert-butyl 4-(2-formylphenyl)piperazine-1-carboxylate scaffold for improved biological activity and target engagement.
Reviews
Write Your Own Review