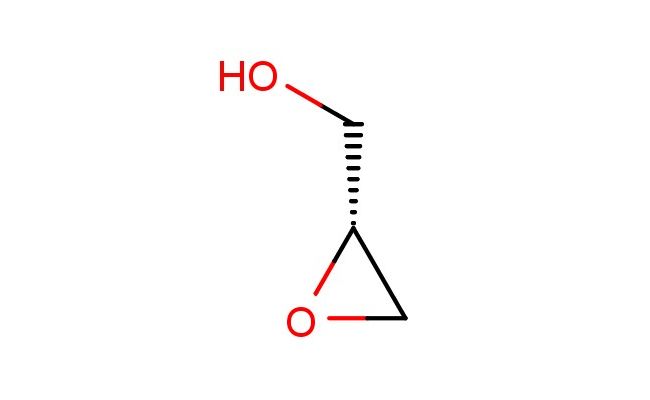
(R)-oxiran-2-ylmethanol
$250.00
CAS No.: 57044-25-4
Catalog No.: 195009
Purity: 95%
MF: C3H6O2
MW: 74.079
Storage: 2-8 degree Celsius
SMILES: O1[C@@H](C1)CO
Catalog No.: 195009
Purity: 95%
MF: C3H6O2
MW: 74.079
Storage: 2-8 degree Celsius
SMILES: O1[C@@H](C1)CO
For R&D use only. Not for human or animal use.
(R)-oxiran-2-ylmethanol; CAS No.: 57044-25-4; (R)-oxiran-2-ylmethanol. PROPERTIES: (R)-oxiran-2-ylmethanol appears as a colorless to pale yellow liquid with a slight ethereal odor. Its molecular formula is C4H7O2, corresponding to a molecular weight of approximately 87.09 g/mol. The compound exhibits a boiling point around 120-122 C at 760 mmHg and a density of about 0.98 g/cm? at 25 C. It demonstrates moderate solubility in water and is miscible with common organic solvents such as ethanol, acetone, and diethyl ether. The substance is sensitive to strong bases and Lewis acids, which may cause ring-opening reactions. Proper storage requires keeping it in a tightly sealed, amber glass container in a cool, dry location away from direct sunlight and heat sources. The temperature should be maintained below 10 C if possible. Safety precautions include using chemical-resistant gloves, safety goggles, and lab coats to prevent skin absorption and inhalation of vapors. The compound may cause skin and eye irritation, and prolonged exposure can lead to central nervous system depression. In case of contact, immediate rinsing with water and medical consultation is recommended. APPLICATIONS: (R)-oxiran-2-ylmethanol serves as a chiral intermediate in asymmetric synthesis, particularly valuable in the preparation of enantiomerically pure compounds used in pharmaceuticals and agrochemicals (Journal of Organic Chemistry). In polymer chemistry, it functions as a building block for creating chiral polycarbonates and epoxy resins with specific optical properties (Macromolecular Chemistry and Physics). Additionally, (R)-oxiran-2-ylmethanol finds application in the synthesis of chiral ligands and catalysts used in transition metal-catalyzed reactions, where its oxirane ring undergoes selective ring-opening to form desired stereochemical configurations (Tetrahedron: Asymmetry). The compound is also utilized in the production of chiral auxiliaries for asymmetric induction in organic transformations, enabling the synthesis of bioactive molecules with high enantiomeric excess (Synthesis).
Reviews
Write Your Own Review