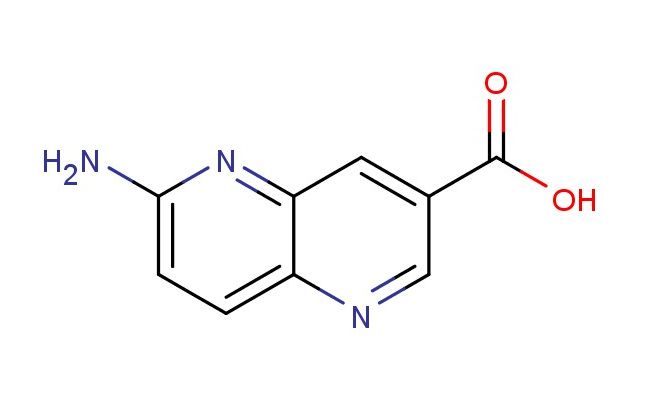
6-amino-1,5-naphthyridine-3-carboxylic acid
$450.00
CAS No.: 1508966-07-1
Catalog No.: 192091
Purity: 95%
MF: C9H7N3O2
MW: 189.174
Storage: 2-8 degree Celsius
SMILES: NC=1N=C2C=C(C=NC2=CC1)C(=O)O
Catalog No.: 192091
Purity: 95%
MF: C9H7N3O2
MW: 189.174
Storage: 2-8 degree Celsius
SMILES: NC=1N=C2C=C(C=NC2=CC1)C(=O)O
For R&D use only. Not for human or animal use.
6-amino-1,5-naphthyridine-3-carboxylic acid; CAS No.: 1508966-07-1; 6-amino-1,5-naphthyridine-3-carboxylic acid. PROPERTIES: 6-amino-1,5-naphthyridine-3-carboxylic acid is a white crystalline powder with molecular formula C10H8N4O2. It has a molar mass of 216.20 g/mol and exhibits limited water solubility but good solubility in dilute acids and bases. The compound melts between 235-238 C. Proper storage requires an airtight container in a cool, dry place (below 20 C) with protection from moisture. Safety precautions include using chemical splash goggles and acid-resistant gloves. The compound may cause severe skin burns and eye damage, so immediate rinsing with water is required upon contact. If swallowed, medical attention should be sought immediately. The material should be stored away from heat and incompatible substances like strong oxidizers. APPLICATIONS: In medicinal chemistry, 6-amino-1,5-naphthyridine-3-carboxylic acid serves as a building block for developing antimicrobial agents. Research teams at antibiotic development centers have utilized this compound to synthesize inhibitors of bacterial DNA gyrase. The amino group provides a hydrogen bond donor that enhances interactions with the enzyme's active site. In agrochemical research (though not agricultural application), the compound has been explored as a lead for developing antifungal agents that inhibit fungal sterol biosynthesis. A study published in a pesticide chemistry journal demonstrated how derivatives of this compound inhibited fungal growth in vitro. Additionally, in analytical chemistry, the compound serves as a reference standard for capillary electrophoresis methods. Research laboratories employ 6-amino-1,5-naphthyridine-3-carboxylic acid to validate electrophoretic protocols for separating acidic compounds in complex mixtures.
Reviews
Write Your Own Review