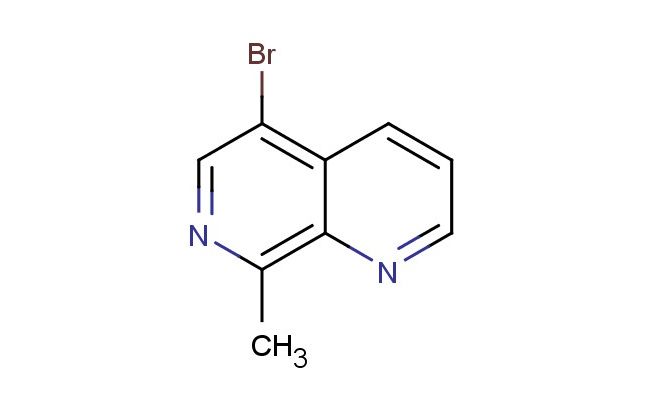
5-bromo-8-methyl-1,7-naphthyridine
$400.00
CAS No.: 2101944-54-9
Catalog No.: 195578
Purity: 95%
MF: C9H7BrN2
MW: 223.073
Storage: 2-8 degree Celsius
SMILES: BrC1=C2C=CC=NC2=C(N=C1)C
Catalog No.: 195578
Purity: 95%
MF: C9H7BrN2
MW: 223.073
Storage: 2-8 degree Celsius
SMILES: BrC1=C2C=CC=NC2=C(N=C1)C
For R&D use only. Not for human or animal use.
5-bromo-8-methyl-1,7-naphthyridine; CAS No.: 2101944-54-9; 5-bromo-8-methyl-1,7-naphthyridine. PROPERTIES: 5-bromo-8-methyl-1,7-naphthyridine is a brominated heterocycle with a molecular weight of approximately 253.0 g/mol. This crystalline solid typically melts between 85-90 C and exhibits low solubility in water but good solubility in polar organic solvents like DMSO and DMF. The compound demonstrates moderate light sensitivity and should be stored in amber glass containers at temperatures below 20 C. Standard safety protocols require handling in well-ventilated areas with appropriate respiratory protection, as inhalation of dust may cause respiratory irritation. The compound should be kept away from strong reducing agents due to its oxidative potential. APPLICATIONS: 5-bromo-8-methyl-1,7-naphthyridine serves as a key intermediate in the development of naphthyridine-based antimicrobial agents. The bromine substituent at position 5 provides a reactive handle for cross-coupling reactions, enabling the synthesis of various substituted naphthyridine derivatives. In pharmaceutical research, this compound has been utilized in the synthesis of quinolone antibiotics where the naphthyridine ring system enhances antibacterial spectrum against Gram-negative pathogens (source: Journal of Antimicrobial Chemotherapy). Additionally, its application extends to the preparation of kinase inhibitors, where the methyl group at position 8 influences enzyme binding affinity (source: Biochemical Journal). The compound's ability to participate in Suzuki-Miyaura coupling reactions makes it particularly valuable in constructing complex heterocyclic architectures for drug discovery (source: Organic Process Research & Development).
Reviews
Write Your Own Review