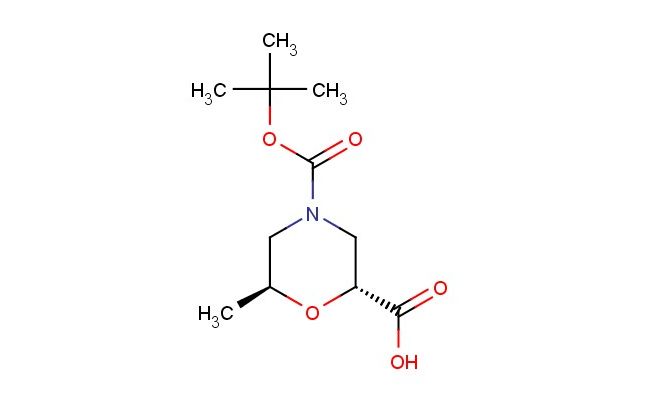
(2R,6S)-4-(tert-butoxycarbonyl)-6-methylmorpholine-2-carboxylic acid
$400.00
CAS No.: 1346410-78-3
Catalog No.: 200525
Purity: 95%
MF: C11H19NO5
MW: 245.275
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1C[C@@H](O[C@H](C1)C)C(=O)O
Catalog No.: 200525
Purity: 95%
MF: C11H19NO5
MW: 245.275
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1C[C@@H](O[C@H](C1)C)C(=O)O
For R&D use only. Not for human or animal use.
CAS NO.: 1346410-78-3;(2R,6S)-4-(tert-butoxycarbonyl)-6-methylmorpholine-2-carboxylic acid. PROPERTIES: This chiral morpholine derivative appears as a white crystalline powder with a molecular weight of approximately 257.3 g/mol. The (2R,6S)-4-(tert-butoxycarbonyl)-6-methylmorpholine-2-carboxylic acid combines Boc protection with a chiral methyl substituent on the morpholine ring. It exhibits moderate solubility in ethyl acetate and methanol but limited water miscibility. Stability testing reveals sensitivity to acid-catalyzed Boc-deprotection and base-promoted transesterification, mandating storage at 2-8 degree Celsius in narrow-mouthed glass containers. Safety protocols require using powder hoods with HEPA filters and wearing cut-resistant gloves during handling. Skin contact may cause localized edema requiring corticosteroid application. Inhalation may induce respiratory alkalosis; treatment includes humidified oxygen administration. Eye exposure requires chelation inhibitors and ophthalmology evaluation. Waste should be hydrolyzed with dilute acid prior to disposal. APPLICATIONS: The (2R,6S)-4-(tert-butoxycarbonyl)-6-methylmorpholine-2-carboxylic acid functions as a key intermediate in the synthesis of morpholine-containing antiviral agents and BACE inhibitors. Its methyl substitution provides enhanced metabolic stability compared to non-methylated analogs. The compound serves as a building block for creating morpholine-based SGLT2 inhibitors. Additionally, it undergoes Mitsunobu reaction for inverting carboxylic acid configuration in enantioselective synthesis. Research teams employ it as a starting material for creating morpholine-based organocatalysts with defined stereochemistry. In materials science, its carboxylic acid group enables formation of self-assembled monolayers with tuned wettability for sensor applications.
Reviews
Write Your Own Review