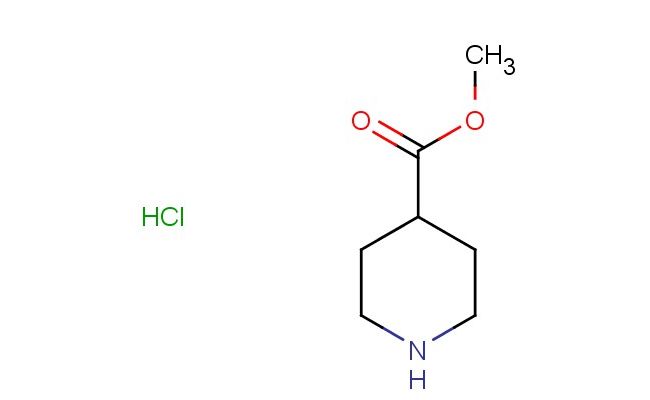
methyl piperidine-4-carboxylate hydrochloride
$250.00
CAS No.: 7462-86-4
Catalog No.: WLZ1370
Purity: 95%
MF: C7H14ClNO2
MW: 179.647
Storage: 2-8 degree Celsius
SMILES: Cl.N1CCC(C(=O)OC)CC1
Catalog No.: WLZ1370
Purity: 95%
MF: C7H14ClNO2
MW: 179.647
Storage: 2-8 degree Celsius
SMILES: Cl.N1CCC(C(=O)OC)CC1
For R&D use only. Not for human or animal use.
CAS NO.: 7462-86-4; methyl piperidine-4-carboxylate hydrochloride. PROPERTIES: This protonated ester features a methyl ester group attached to a piperidine ring bearing a carboxylic acid functionality at the 4-position, creating a molecule with potential applications in medicinal chemistry and pharmaceutical research. The methyl piperidine-4-carboxylate hydrochloride typically appears as a white crystalline powder with high aqueous solubility due to its salt form. Its molecular structure includes a quaternary ammonium center that imparts water solubility and influences biological membrane permeability. For optimal stability and to preserve its crystalline form, this compound should be stored at 2-8 degree Celsius in a tightly sealed container under anhydrous conditions. When handling, appropriate safety measures including nitrile gloves and safety goggles are essential. This compound is hygroscopic and may absorb moisture from the atmosphere. In case of accidental spillage, clean the area with a damp cloth and dispose of materials according to local regulations. APPLICATIONS: The methyl piperidine-4-carboxylate hydrochloride serves as a valuable intermediate in the synthesis of pharmaceuticals, particularly those targeting the central nervous system. The piperidine ring provides a platform for developing receptor modulators with specific binding profiles. In medicinal chemistry, this compound functions as a building block for creating antipsychotic and anxiolytic agents where the ester functionality can be further modified for improved pharmacokinetic properties. Additionally, the molecule finds utility in chemical biology studies where its defined structure can be used to probe enzyme inhibition mechanisms and cellular signaling pathways. Researchers utilizing this compound benefit from its functional group compatibility, enabling the development of therapeutic agents targeting various disease pathways including neuropsychiatric conditions.
Reviews
Write Your Own Review