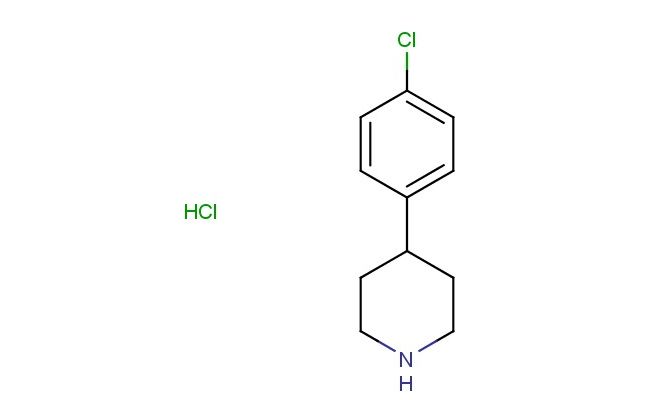
4-(4-chlorophenyl)piperidine hydrochloride
$200.00
CAS No.: 6652-06-8
Catalog No.: WLZ1381
Purity: 95%
MF: C11H15Cl2N
MW: 232.154
Storage: 2-8 degree Celsius
SMILES: Cl.ClC1=CC=C(C=C1)C1CCNCC1
Catalog No.: WLZ1381
Purity: 95%
MF: C11H15Cl2N
MW: 232.154
Storage: 2-8 degree Celsius
SMILES: Cl.ClC1=CC=C(C=C1)C1CCNCC1
For R&D use only. Not for human or animal use.
CAS NO.: 6652-06-8; 4-(4-chlorophenyl)piperidine hydrochloride. PROPERTIES: This protonated amine features a piperidine ring substituted with a 4-chlorophenyl group, creating a molecule with potential applications in medicinal chemistry and pharmaceutical research. The 4-(4-chlorophenyl)piperidine hydrochloride typically appears as a white crystalline powder with high aqueous solubility due to its salt form. Its molecular structure includes a chlorinated aromatic moiety that influences lipophilicity and receptor binding characteristics. For optimal stability and to preserve its crystalline form, this compound should be stored at 2-8 degree Celsius in a tightly sealed container under anhydrous conditions. When handling, appropriate safety measures including nitrile gloves and safety goggles are essential. This compound is hygroscopic and may absorb moisture from the atmosphere. In case of accidental spillage, clean the area with a damp cloth and dispose of materials according to local regulations. APPLICATIONS: The 4-(4-chlorophenyl)piperidine hydrochloride serves as a valuable intermediate in the synthesis of pharmaceuticals, particularly those targeting the central nervous system and cardiovascular conditions. The piperidine ring provides a platform for developing receptor modulators with specific binding profiles. In medicinal chemistry, this compound functions as a building block for creating antipsychotic and antidepressant agents where the chlorophenyl group contributes to target engagement and selectivity. Additionally, the molecule finds utility in chemical biology studies where its defined structure can be used to probe enzyme inhibition mechanisms and cellular signaling pathways. Researchers utilizing this compound benefit from its functional group compatibility, enabling the development of therapeutic agents targeting various disease pathways including neuropsychiatric and cardiovascular conditions.
Reviews
Write Your Own Review